Rare-earth element facts for kids
Rare-earth elements (REEs) are a group of 17 special metals. They are shiny, silvery-white, and soft. Even though they are called "rare," they are actually quite common in the Earth's crust. The name "rare-earth" comes from how hard they were to find and separate a long time ago.
These elements include scandium, yttrium, and the 15 lanthanides. Scandium and yttrium are grouped with them because they are often found in the same places and act in similar ways. Rare-earth elements are super important for many modern technologies, from phones to electric cars.
Contents
- What are Rare-Earth Elements?
- Meet the Rare-Earth Elements
- How Rare-Earth Elements Were Discovered
- Where Do Rare-Earth Elements Come From?
- How We Use Rare-Earth Elements Today
- Environmental Concerns with Rare-Earth Elements
- Recycling Rare-Earth Elements
- Global Supply of Rare-Earth Elements
- Images for kids
- See also
What are Rare-Earth Elements?
Rare-earth elements are a group of 17 metallic chemical elements. They are known for their unique properties. These properties make them very useful in many high-tech products. They are often found together in the same types of rocks.
Why are they called "rare"?
The name "rare-earth" is a bit misleading. These elements are not actually scarce in the Earth's crust. For example, cerium is more common than copper. However, they are usually spread out in tiny amounts. It is very expensive and difficult to gather them in large, pure amounts. This is why they got the name "rare."
How do rare earths behave?
These metals slowly lose their shine in the air. They also react slowly with cold water. If they get hot enough (around 400°C or 752°F), they can catch fire. Most rare earths and their compounds do not have a role in living things. Some are used in special enzymes found in bacteria. The ones that dissolve in water can be a little harmful. But those that do not dissolve are usually safe.
Promethium is one rare-earth element that is different. All its forms are radioactive. It is not found naturally on Earth. Only tiny traces come from the natural breakdown of uranium-238.
Meet the Rare-Earth Elements
Here is a list of the 17 rare-earth elements. It shows their atomic number, symbol, and what their names mean. It also lists some of their main uses. Many names come from places or scientists.
| Z | Symbol | Name | What its name means | Main uses | Amount in Earth's crust (parts per million) |
|---|---|---|---|---|---|
| 21 | Sc | Scandium | From Latin Scandia (Scandinavia). | Light alloys for airplanes, special lamps, tracing agents in oil refineries. | 22 |
| 39 | Y | Yttrium | Named after Ytterby, Sweden, where the first rare-earth ore was found. | Lasers, red color in old TVs, superconductors, strong ceramics for jet engines and tooth crowns, energy-saving light bulbs, spark plugs, cancer treatments. | 33 |
| 57 | La | Lanthanum | From Greek "lanthanein," meaning to be hidden. | Special glass for cameras, hydrogen storage, battery parts, catalysts for oil refineries. | 39 |
| 58 | Ce | Cerium | After the dwarf planet Ceres, named after the Roman goddess of farming. | Cleaning agents, polishing powders, yellow colors in glass, catalysts for self-cleaning ovens, flints for lighters. | 66.5 |
| 59 | Pr | Praseodymium | From Greek "prasios" (leek-green) and "didymos" (twin). | Strong magnets, lasers, lighting, colors in glass and enamel, special glass for welding goggles. | 9.2 |
| 60 | Nd | Neodymium | From Greek "neos" (new) and "didymos" (twin). | Strong magnets, lasers, violet colors in glass, electric motors in electric cars. | 41.5 |
| 61 | Pm | Promethium | After the Titan Prometheus, who brought fire to humans. | Nuclear batteries, glowing paint. | 1×10−15 |
| 62 | Sm | Samarium | After mine official, Vasili Samarsky-Bykhovets. | Strong magnets, lasers, absorbing neutrons in nuclear reactors. | 7.05 |
| 63 | Eu | Europium | After the continent of Europe. | Red and blue glowing materials (phosphors), lasers, mercury lamps. | 2 |
| 64 | Gd | Gadolinium | After Johan Gadolin, who studied rare earths. | Special glass, lasers, X-ray tubes, MRI scans, steel alloys, magnetic cooling. | 6.2 |
| 65 | Tb | Terbium | After the village of Ytterby, Sweden. | Magnets, green glowing materials, lasers, special alloys for sonar systems. | 1.2 |
| 66 | Dy | Dysprosium | From Greek "dysprositos," meaning hard to get. | Magnets, lasers, special alloys for hard disk drives. | 5.2 |
| 67 | Ho | Holmium | After Stockholm (in Latin, "Holmia"). | Lasers, standards for measuring light, magnets. | 1.3 |
| 68 | Er | Erbium | After the village of Ytterby, Sweden. | Infrared lasers, special steel, fiber-optic technology. | 3.5 |
| 69 | Tm | Thulium | After the mythical northern land of Thule. | Portable X-ray machines, special lamps, lasers. | 0.52 |
| 70 | Yb | Ytterbium | After the village of Ytterby, Sweden. | Infrared lasers, chemical reducing agent, decoy flares, stainless steel, nuclear medicine. | 3.2 |
| 71 | Lu | Lutetium | After Lutetia, the city that later became Paris. | PET scan detectors, high-refractive-index glass, catalysts in oil refineries, LED light bulbs. | 0.8 |
How Rare-Earth Elements Were Discovered
The story of rare-earth discovery is long and full of challenges. Scientists had a hard time separating them. This was because they are so similar in their chemical properties.
Early discoveries
The first rare-earth mineral was found in 1787. It was called "ytterbite" and was discovered in a mine in Ytterby, Sweden. A scientist named Johan Gadolin studied it. He found an unknown substance he called "yttria." Later, in 1803, other scientists found a new oxide called "ceria" from a different mineral.
For many years, scientists thought these "earths" were single elements. But they were actually mixtures. It took a lot of work to separate them.
In 1839, Carl Gustav Mosander managed to separate ceria. He found a new oxide he called "lanthana," meaning to be hidden. He later separated lanthana into "didymia" and pure lanthana. Mosander also separated yttria into three parts: pure yttria, terbia, and erbia. By 1842, six rare-earth elements were known.
Because they were so hard to separate, many scientists made mistakes. They thought they had found new elements when they hadn't.
Using light to find new elements
About 30 years later, scientists started using a new method. It was called atomic emission spectroscopy. This method looks at the light elements give off when heated. In 1879, Marc Delafontaine found new lines in didymia's light. This showed that didymia was still a mixture.
That same year, Paul Émile Lecoq de Boisbaudran found a new element called samarium. Later, he and others found gadolinium and europium using similar methods.
The atomic number breakthrough
For a long time, no one knew exactly how many rare-earth elements existed. Some thought there could be up to 25! Then, Henry Moseley used X-ray technology. He found that there should be exactly 15 lanthanides. He also predicted that element 61, promethium, was still missing. Promethium was later found, but it is radioactive and does not last long.
Moseley's work also showed that hafnium (element 72) was not a rare-earth element. This helped clear up a lot of confusion.
Where Do Rare-Earth Elements Come From?
Rare-earth elements are found in certain minerals. The main ones are bastnäsite, monazite, and ion-adsorption clays. Even though they are common, getting them out of the ground is tough. This is because they are often mixed together.
Mining and purification
Before the 1940s, most rare earths came from sand deposits in India and Brazil. Later, mines in South Africa and the United States became important. During the 1940s, new methods like ion exchange were developed. These methods made it much easier to separate and purify rare-earth elements. This made them more useful for industry.
Types of rare-earth elements
Scientists classify rare-earth elements into two main groups:
- Light Rare-Earth Elements (LREE): These have lower atomic numbers (like lanthanum to promethium).
- Heavy Rare-Earth Elements (HREE): These have higher atomic numbers (like samarium to lutetium). Yttrium is also grouped with HREEs because it acts similarly.
This classification is important because LREEs and HREEs are found in different amounts. HREEs are harder to find in large, concentrated deposits.
How We Use Rare-Earth Elements Today
Rare-earth elements are essential for many modern technologies. Their uses have grown a lot over the years.
Everyday uses
Globally, most rare-earth elements are used in catalysts and magnets. Catalysts help speed up chemical reactions. They are used in petroleum refining and in diesel additives for cars.
Magnets made with rare earths are super strong. They are found in:
- Electric motors for hybrid and electric vehicles.
- Generators in some wind turbines.
- Hard disk drives in computers.
- Portable electronics like smartphones.
- Microphones and speakers.
Other important uses
Rare earths are also used in:
- Alloys: Mixing metals to make them stronger or give them special properties.
- Batteries: Especially nickel-metal hydride batteries.
- Electronics: For LCD and plasma screens, fiber optics, and lasers.
- Medical imaging: Like MRI scans.
- Glass: To make it stronger or give it special colors.
- Polishing: For very smooth surfaces.
- Lighting: In fluorescent lamps and LEDs.
Some rare earths have even been used in farming. They are added to fertilizers to help plants grow better. They have also been used in animal feed to help livestock grow. However, scientists are still studying the long-term effects of this.
Environmental Concerns with Rare-Earth Elements
Mining and processing rare-earth elements can cause environmental problems. This is especially true if it is not done carefully.
Pollution from mining
Rare-earth ores often contain small amounts of radioactive materials like thorium and uranium. If these are not handled properly, they can cause pollution. Waste from mines can release toxic materials into water and soil. This can harm plants, animals, and even people.
For example, strong acids are used to extract rare earths. These acids can leak into the environment. Also, cerium oxide is released from diesel exhaust. This adds to soil and water pollution.
Impact on living things
When rare earths get into the soil and water, they can be absorbed by plants. Some plants absorb more than others. If animals or humans eat these plants, the rare earths can build up in their bodies. This is called bioaccumulation.
Studies have shown that high levels of rare earths can harm plants. They can reduce chlorophyll, which plants need for photosynthesis. For animals, high levels can affect organs like the lungs and liver.
Health effects on humans
People living near rare-earth mines can have higher levels of these elements in their bodies. This can happen from breathing in dust or drinking contaminated water. While more research is needed, some studies suggest possible health issues. These might include breathing problems or effects on brain development in children.
It is important for mining companies to follow strict safety rules. They need to clean up waste properly to protect the environment and people's health.
Recycling Rare-Earth Elements
Recycling rare-earth elements is becoming very important. This helps reduce the need for new mining. It also helps protect the environment.
Why recycle?
Currently, only a small amount of rare earths are recycled from old products. Most end up in landfills. Recycling helps:
- Reduce environmental pollution from mining.
- Save valuable resources.
- Lower the cost of getting rare earths.
How are they recycled?
Scientists are working on new ways to recycle rare earths. One method involves using special bacteria. These bacteria can dissolve and separate rare earths from electronic waste.
Electronic waste, like old phones and computers, is a big source of rare earths. About 50 million tons of e-waste are thrown away each year. Only a small part of this is recycled.
Challenges in recycling
One big challenge is separating the rare earths from each other. They have very similar chemical properties. New technologies are needed to make this process easier and cheaper.
Global Supply of Rare-Earth Elements
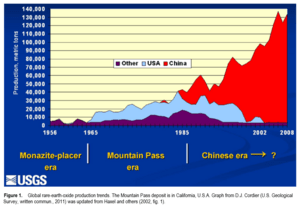
For many years, China has been the world's main supplier of rare-earth elements. In 2017, China produced 81% of the world's supply. This has led to some concerns about supply.
China's role
China has reduced its rare-earth exports over the years. They say this is to protect their resources and the environment. However, some believe it is also to help Chinese companies make more finished products. This way, China can sell valuable goods instead of just raw materials.
This situation has caused other countries to look for their own sources.
Other countries seeking rare earths
Many countries are now trying to find and develop their own rare-earth mines. These include Australia, Brazil, Canada, South Africa, Tanzania, Greenland, and the United States. Some mines that closed in the past are now being reopened.
For example, the Mountain Pass rare earth mine in California, USA, has been mined on and off since 1951. In 2023, Sweden announced a discovery of a large rare-earth deposit. This could be the biggest in Europe.
Some countries are also looking into getting rare earths from other sources. These include:
- Mine tailings: Waste from old mines can still contain valuable rare earths.
- Ocean mining: Deep-sea mud on the ocean floor has been found to contain rich amounts of rare earths.
- Electronic waste: Recycling old electronics is a growing source.
The demand for rare earths is expected to keep growing. This is because they are so important for new technologies. Finding new sources and improving recycling will be key for the future.
Images for kids
See also
 In Spanish: Tierras raras para niños
In Spanish: Tierras raras para niños
 | Roy Wilkins |
 | John Lewis |
 | Linda Carol Brown |