Paul-Émile Lecoq de Boisbaudran facts for kids
Quick facts for kids
Paul-Émile Lecoq de Boisbaudran
|
|
|---|---|
 |
|
| Born | 18 April 1838 |
| Died | 28 May 1912 (aged 74) Paris, France
|
| Known for | Discovery of gallium, samarium and dysprosium Contributions to spectroscopy |
| Awards | Davy Medal (1879) |
| Scientific career | |
| Fields | Chemistry, spectroscopy |
Paul-Émile Lecoq de Boisbaudran (born April 18, 1838, died May 28, 1912) was a French chemist. He is famous for finding three new chemical elements: gallium, samarium, and dysprosium. He also helped create new ways to separate and clean rare earth elements. He was a leader in the science of spectroscopy, which studies how light and matter interact.
Contents
About Paul-Émile Lecoq de Boisbaudran
Paul-Émile Lecoq de Boisbaudran came from a noble French family called the Huguenots. Huguenots were French Protestants who faced tough times in France. Many of their family's belongings were taken away a long time ago.
Paul-Émile's father, Paul Lecoq de Boisbaudran, started a successful wine business in Cognac. Young Paul-Émile helped his family with this business. His mother, Anne Louise, was very educated. She taught Paul-Émile history and languages like English.
Paul-Émile taught himself science by studying books from a famous French school called the École Polytechnique. This means he was an autodidact, someone who learns on their own. With his family's help, he set up a small chemical laboratory at home. In this lab, he repeated experiments he read about. This is how he developed his ideas about spectroscopy and found most of his discoveries, including gallium.
Paul-Émile Lecoq de Boisbaudran married Jeannette Nadault-Valette in 1897. They did not have any children. After 1895, a health problem that made it hard for him to move his joints slowed down his work. He passed away in 1912 when he was 74 years old.
His Scientific Discoveries
Lecoq de Boisbaudran did important work in spectroscopy. This is a science that looks at how light and matter interact. He used spectroscopy to study and identify different chemical elements, especially the rare-earth elements. He believed that the light patterns of elements were connected to their atomic weight.
He created new tools and used them to study the light patterns of 35 different elements. He used a Bunsen burner or electric sparks to make the elements glow. He wrote about his early findings in a book in 1874.
To see the light patterns, he often put a salt solution in a glass tube. He used platinum wires to create an electric spark. Later, he changed the direction of the electric current. This helped him see even more details in the light patterns. Using these methods, he discovered samarium (in 1880), dysprosium (in 1886), and europium (in 1890). He also studied gadolinium in 1885, which another scientist had found earlier.
Discovery of Samarium
Lecoq de Boisbaudran found samarium in 1879. He first separated samarium oxide. He used spectroscopy to see its special light patterns, which showed him a new element was present.
He named this new element "samarium" after the mineral samarskite. This mineral was named after a Russian mining official, Colonel Vassili Samarsky-Bykhovets.
Discovery of Gallium
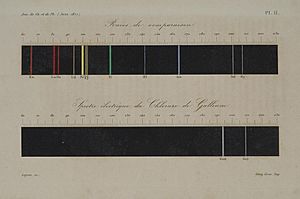
One of Lecoq de Boisbaudran's biggest achievements was finding the element gallium in 1875. He started by studying a large amount of a mineral called sphalerite. From this, he got a tiny bit of gallium chloride. When he used spectroscopy, he saw two light lines that no one had ever reported before.
He kept working with even more raw zinc ore. In the same year, he was able to get more than one gram of almost pure gallium. He used electricity to separate it from a solution. Later, he made 75 grams of gallium from over 4 tons of raw ore. He checked its light patterns again. This proved that the special light lines were from a new element, not just a mistake in his process.
He named his discovery "gallium" after Gallia, the Latin name for Gaul, which is France. Some people thought he named it after himself, because gallus is Latin for "le coq" (his last name). But he said this was not true. He wrote about his discovery in a paper in 1877.
Lecoq de Boisbaudran calculated gallium's atomic mass to be 69.86. This is very close to the actual value today, which is 69.723. What he didn't know was that another scientist, Dmitri Mendeleev, had already predicted gallium's existence in 1871. Mendeleev had called it eka-aluminium. Lecoq de Boisbaudran's discovery of gallium was a big help in proving Mendeleev's idea of the periodicity of the elements, which is how elements are organized in the periodic table.
Discovery of Dysprosium
Lecoq de Boisbaudran also experimented with separating rare earth compounds from water. He noticed a special light band in the yellow-green part of the spectrum. This suggested a new element. In 1886, he successfully separated a pure sample of the source of this new light band. He named the element dysprosium. This name means "difficult to obtain" in Greek, because it was very hard to get.
Periodic Table Contributions
Lecoq de Boisbaudran also helped with the development of the periodic table of elements. He suggested that the newly found element argon belonged to a new group of elements. This group later became known as the noble gases.
Awards and Recognition
For his important work, Lecoq de Boisbaudran received several awards:
- The Cross of the Legion of Honour (1876)
- The Bordin Prize from the French Academy of Sciences (1872)
- The Davy Medal (1879)
- The Prix Lacaze of 10,000 francs (1879)
In 1888, he was also chosen as a foreign member of the British Royal Society.
Images for kids
See also
 In Spanish: Paul Émile Lecoq de Boisbaudran para niños
In Spanish: Paul Émile Lecoq de Boisbaudran para niños