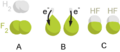Reduction (chemistry) facts for kids
Reduction is a special kind of chemical reaction where an atom or molecule gains electrons. Think of electrons as tiny, negatively charged particles that are part of every atom. When an atom gains electrons, its "oxidation state" (which is like a score that tells us how many electrons it has gained or lost) goes down. That's why it's called "reduction"—because its oxidation state is reduced!
Reduction usually happens at the same time as another reaction called oxidation. When one substance gains electrons (reduction), another substance must lose them (oxidation). These two reactions together are called a "redox" reaction.
A common example of reduction is when iron reacts with oxygen to form rust. In this process, the iron loses electrons (it gets oxidized), and the oxygen gains electrons (it gets reduced). So, rust is a result of a redox reaction!
Contents
What is Reduction?
Reduction is all about gaining electrons. Imagine atoms like tiny banks that can give or take electrons. When an atom "reduces," it's like it's getting more money (electrons) into its account. This makes its "oxidation state" number smaller. For example, if an atom has an oxidation state of +2 and gains two electrons, its new oxidation state might become 0.
Reduction and Oxidation: A Team Effort
You almost never find reduction happening by itself. It's always paired with oxidation.
- Reduction means gaining electrons.
- Oxidation means losing electrons.
It's like a trade: one atom gives electrons, and another atom takes them. This is why these reactions are called "redox" reactions – it's a mix of "red" (from reduction) and "ox" (from oxidation).
Rust: A Common Redox Example
When you see rust on a metal object, you're seeing a redox reaction in action.
- The iron metal loses electrons to the oxygen. This means the iron is oxidized.
- The oxygen gains those electrons from the iron. This means the oxygen is reduced.
So, rust forms because iron and oxygen are trading electrons!
Reduction in Industry
Reduction reactions are very important in many industries. For example, to make new iron from iron ore (which is basically iron that has already rusted), we need to reverse the rusting process. This is done in huge ovens called blast furnaces.
- In a blast furnace, carbon monoxide is used as a "reducing agent". This means it helps the iron ore gain back its electrons.
- The carbon monoxide takes the oxygen away from the iron, leaving pure iron metal. This is a big reduction reaction that helps us get the metals we use every day.
Related pages
Images for kids
-
Sodium and fluorine bonding ionically to form sodium fluoride. Sodium loses its outer electron to give it a stable electron configuration, and this electron enters the fluorine atom exothermically. The oppositely charged ions are then attracted to each other. The sodium is oxidized; and the fluorine is reduced.
-
Oxides, such as iron(III) oxide or rust, which consists of hydrated iron(III) oxides Fe2O3·nH2O and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3), form when oxygen combines with other elements
-
Iron rusting in pyrite cubes
See also
 In Spanish: Reducción-oxidación para niños
In Spanish: Reducción-oxidación para niños
 | William M. Jackson |
 | Juan E. Gilbert |
 | Neil deGrasse Tyson |