Blast furnace facts for kids
A blast furnace is a huge, special type of furnace used to make iron from iron ore. Think of it as a giant oven! These furnaces are very tall, sometimes as high as 60 metres (200 ft) (about 200 feet) and 15 metres (49 ft) (about 50 feet) wide. They are also known as high ovens.
A blast furnace is usually built with a strong steel outer shell. Inside, it has special bricks made from materials like magnesium oxide. These bricks can handle extremely high temperatures without melting. To keep the furnace from getting too hot, water flows through parts of its walls.
The main goal of a blast furnace is to turn iron ore into usable iron. Iron ore is mostly iron oxide, which means iron mixed with oxygen. The furnace works by removing this oxygen. This process is called smelting. The result is a type of crude iron called pig iron. To do this, carbon (usually from a fuel called coke) is heated to very high temperatures with the ore. The carbon easily takes the oxygen away from the iron ore.
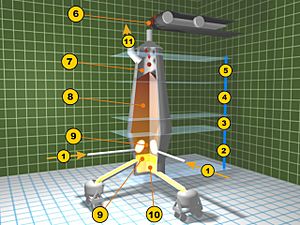
1. Hot air ("wind") from Cowper stoves
2. Melting zone
3. Reduction zone of iron oxide
4. Another reduction zone
5. Pre-heating zone
6. Where ore, limestone, and coke are added
7. Exhaust gases leave
8. Column of materials inside
9. Slag is removed
10. Molten pig iron is tapped
11. Waste gases are collected
Contents
How a Blast Furnace Works
Adding Materials
To start the process, iron ore, limestone, and a type of carbon called coke are loaded into the very top of the blast furnace. These materials are added in layers.
The "Blast" of Air
At the same time, very hot air (called "wind") is blown into the furnace. This hot air enters through special openings called "tuyeres" located at the bottom of the furnace. This action of blowing hot air is why it's called a "blast furnace"!
Burning and Reactions
The coke inside the furnace catches fire and burns. Because there isn't enough oxygen to make carbon dioxide, the burning coke creates carbon monoxide. This carbon monoxide is super important! It reacts with the iron oxide in the ore, taking away the oxygen and leaving behind pure iron. This process also creates carbon dioxide. The limestone added to the furnace helps to collect impurities (unwanted rock parts) from the iron ore, forming a substance called slag.
Removing Iron and Slag
The bottom part of the furnace is called the hearth. As the iron and slag melt, they collect here as liquids. Slag is lighter than iron and floats on top, like cream on milk.
First, the liquid slag is removed. This is called skimming. A special drill makes a hole in the hearth at the slag's level. The liquid slag then flows out into a special container called a slag pot.
After the slag is removed, the liquid pig iron is drained. This is called tapping. Another hole is made at the very bottom of the furnace, and the molten pig iron flows out. This pig iron can be used right away to make steel, or it can be transported in special railway wagons called torpedo cars, or poured into molds to cool. Once all the iron is out, the holes are quickly sealed with a special type of clay that hardens fast in the heat.
From Pig Iron to Steel
The pig iron that comes out of the blast furnace contains about 4% carbon. This makes it very hard and brittle, meaning it can break easily. To make it useful, most of this extra carbon must be removed. This process is called "decarburizing," and it turns pig iron into steel.
Today, a common way to turn pig iron into steel is using a basic oxygen furnace. In the past, other methods like the Bessemer converter were used.
Using Waste Products
The gases that rise to the top of the furnace are collected. These gases contain a lot of carbon monoxide, which is a valuable fuel. This collected gas is called blast furnace gas. It's cleaned to remove any dust, then burned in special ovens called Cowper stoves or hot blast stoves. The heat from burning this gas is then used to pre-heat the "wind" (hot air) that is blown back into the blast furnace. This makes the whole process more efficient!
The slag is also not wasted! It can be used to make bricks for construction or mixed with concrete. Concrete made with blast furnace slag is often stronger and whiter than regular concrete.
A blast furnace can usually operate continuously for 10 to 20 years without stopping. This long period of operation is called a "campaign."
Chemical Reactions in a Blast Furnace
At very high temperatures (around 900-1600°C), carbon reacts with iron oxides to create pure iron. Here are some of the main reactions:
| 1. | 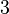 |
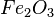   |
 |
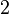 |
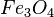 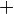 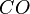 |
| 2. | 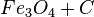 |
 |
 |
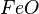 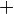 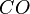 |
|
| 3. | 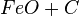 |
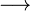 |
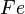 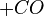 |
These reactions show how oxygen is removed from the iron ore, leaving behind pure iron.
Images for kids
-
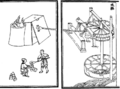
An illustration of furnace bellows operated by waterwheels, from the Nong Shu, by Wang Zhen, 1313, during the Yuan Dynasty of China
-
The original blast furnaces at Blists Hill, Madeley, England
-
Abandoned blast furnace in Sestao, Spain. The furnace itself is inside the central girderwork.
-
Part of the gas cleaning system of a blast furnace in Monclova, Mexico. This one is about to be de-commissioned and replaced.
See also
 In Spanish: Alto horno para niños
In Spanish: Alto horno para niños
 | Lonnie Johnson |
 | Granville Woods |
 | Lewis Howard Latimer |
 | James West |