Electron cloud facts for kids
The electron cloud is a way to imagine where electrons are found around the center of an atom. Think of it like a fuzzy cloud instead of tiny planets orbiting a sun. This idea helps us understand how electrons behave.
This "electron cloud" idea is newer than the older Bohr model of the atom. In the Bohr model, Niels Bohr said electrons move in clear paths or "orbits" around the nucleus. But scientists found that electrons don't really follow exact paths.
The electron cloud model explains that we can't know the exact spot of an electron at any moment. Instead, we can only know where an electron is most likely to be. It's like a blurry picture showing the areas where an electron spends most of its time. The chance of finding an electron gets much smaller the further you look from the nucleus. This is the most accepted way scientists describe atoms today.
In the Bohr model, electrons were placed in different "shells." These shells helped explain why elements in the periodic table have similar chemical properties. With the electron cloud model, scientists use quantum mechanics to place electrons into different "atomic orbitals." These orbitals are not all perfect spheres; they have different shapes. These atomic orbitals also help explain the patterns we see in the periodic table.
The electron cloud model was developed in 1925 by two famous scientists, Erwin Schrödinger and Werner Heisenberg. It helps us picture the most probable places electrons can be in an atom. This model is the one scientists use today to understand atoms.
Contents
Understanding Electron Orbitals
In the electron cloud model, the "cloud" is made of different shapes called atomic orbitals. Each orbital is a region where an electron is most likely to be found. These orbitals have specific shapes and energy levels.
Different Shapes of Orbitals
Electrons can be found in different types of orbitals, each with a unique shape:
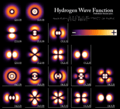
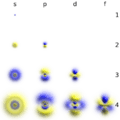
- s orbitals: These are shaped like spheres. The smallest atoms often have electrons in s orbitals.
- p orbitals: These look like two balloons tied together, or a dumbbell shape. There are three p orbitals for each energy level, pointing in different directions (x, y, and z).
- d orbitals: These have more complex shapes, often like four-leaf clovers or two dumbbells with a donut around them.
- f orbitals: These are even more complex and are found in larger atoms.
These shapes are important because they affect how atoms join together to form molecules.
Why the Cloud is Fuzzy
The "fuzziness" of the electron cloud comes from a rule in quantum mechanics called the uncertainty principle. This principle says that you can't know both the exact position and the exact speed of an electron at the same time. If you know where it is, you can't know how fast it's moving, and vice versa.
Because of this, we can only talk about the probability of finding an electron in a certain area. The denser (thicker) parts of the cloud show where the electron is most likely to be. The thinner parts show where it's less likely to be.
Electron Cloud vs. Bohr Model
The Bohr model was a big step forward in understanding atoms, but it had some limits. It worked well for simple atoms like hydrogen but not for more complex ones.
Bohr's Orbits
In the Bohr model, electrons were thought to orbit the nucleus like planets around the sun. Each orbit had a specific energy level. Electrons could jump between these orbits by gaining or losing energy. This explained some things, like how atoms give off light.
Modern View of Electrons
The electron cloud model, based on quantum mechanics, gives a more accurate picture. It doesn't say electrons follow fixed paths. Instead, it describes their behavior using mathematical equations that show probability. This model helps scientists understand chemical reactions and how materials behave much better than the Bohr model. It's the foundation for modern chemistry and physics.
Images for kids
-
The shapes of the first five atomic orbitals are: 1s, 2s, 2px, 2py, and 2pz. The two colors show the phase or sign of the wave function in each region. Each picture is domain coloring of a ψ(x, y, z) function which depend on the coordinates of one electron. To see the elongated shape of ψ(x, y, z)2 functions that show probability density more directly, see pictures of d-orbitals below.
See also
 In Spanish: Orbital atómico para niños
In Spanish: Orbital atómico para niños
 | Chris Smalls |
 | Fred Hampton |
 | Ralph Abernathy |