Bayer process facts for kids
The Bayer process is a super important way that factories make alumina, which is also called aluminium oxide. Alumina is the main ingredient needed to make aluminium metal. This process was invented by a smart person named Carl Josef Bayer.
Aluminium comes from a rock called bauxite. Bauxite isn't pure aluminium oxide; it's usually only about 30% to 60% alumina. The rest is a mix of other stuff like sand, iron rust, and titanium dioxide. The Bayer process helps to clean up the alumina so it can be turned into pure aluminium.
The Bayer process also helps us get a rare metal called gallium. It's a byproduct, meaning it's made along with the main product, even though not much of it is collected.
Contents
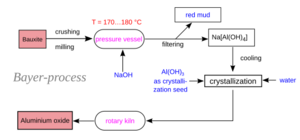
How the Bayer Process Works
Bauxite ore is a mix of aluminium compounds and other elements like iron. The aluminium in bauxite can be in different forms, like gibbsite, böhmite, or diaspore. These different forms and the impurities in the rock change how the process works.
Aluminium compounds can act like both acids and bases. They don't dissolve well in plain water. But they dissolve much better in water that is very acidic or very basic.
In the Bayer process, bauxite ore is heated in a strong container called a pressure vessel. It's mixed with a solution of sodium hydroxide, which is a very strong basic liquid (also called caustic soda). This happens at high temperatures, usually between 150°C and 200°C.
At these hot temperatures, the aluminium in the bauxite dissolves. It forms a new substance called sodium aluminate. The other parts of the bauxite, like iron, do not dissolve.
Digestion and Separation
The heating and dissolving part is called digestion. It turns the aluminium oxide in the bauxite into soluble sodium aluminate. Here's a simplified chemical idea of what happens:
- Al(OH)3 + NaOH → NaAlO2 + 2 H2O
Sometimes, sand (silica) in the bauxite also dissolves. To prevent this from being a problem, sometimes lime is added. This helps the silica turn into a solid that can be filtered out.
After digestion, the liquid is filtered to remove all the solid impurities. These impurities are called red mud. They contain iron, sand, and other stuff that didn't dissolve.
Making Alumina Crystals
The clean liquid, which now has dissolved sodium aluminate, is then cooled down. Small, pure aluminium hydroxide crystals from earlier batches are added. These are called seed crystals. These tiny crystals help the dissolved aluminium hydroxide to come out of the solution and form new, larger crystals. This process can take several days if no seed crystals are added.
Here's the simplified chemical idea of how the aluminium hydroxide crystals form:
- 2 H2O + NaAlO2 → Al(OH)3 + NaOH
Some of the aluminium hydroxide made this way is used to make chemicals for cleaning water. A lot of it is also used as a filler in rubber and plastics, and it can even help stop fires.
Turning Crystals into Alumina
About 90% of the aluminium hydroxide crystals are then heated very strongly. They are heated in special ovens called rotary kilns or calciners to about 1470 Kelvin (which is very hot!). This heating removes the water from the aluminium hydroxide. What's left is pure alumina (aluminium oxide).
The sodium hydroxide solution that is left over after the alumina is made is then reused. This makes the process more efficient and saves money. Reusing the liquid also helps to collect other valuable metals like gallium and vanadium, which can then be taken out.
If bauxite has too much sand (more than 10% silica), the Bayer process doesn't work as well. This is because the sand forms a solid that wastes some of the valuable sodium. In such cases, a different method might be used.
To make about 1 ton of pure alumina, you need about 1.9 to 3.6 tons of bauxite. This is because most of the aluminium in the rock dissolves during the process. The process uses a lot of energy, mostly heat. Over 90% of the alumina made is then used in another process called the Hall–Héroult process to make pure aluminium metal.
Waste Product: Red Mud
Red mud is the main waste product from the Bayer process. It's what's left over after the bauxite is digested with sodium hydroxide. Red mud has a lot of calcium and sodium hydroxide, and it's very basic (alkaline). This means it can be harmful to the environment if not handled carefully.
A lot of red mud is produced, so scientists are always looking for ways to use it. It might even be a source for metals like vanadium. Also, some of the gallium that isn't collected ends up in the red mud or in the alumina itself.
Red mud can be used in making ceramics. When it dries, it becomes a fine powder with iron, aluminium, calcium, and sodium. However, it can be a health risk if not managed properly.
In some places, like the United States, red mud is stored in large storage areas called impoundments. These are like big ponds with special linings to keep the mud from leaking into the ground. Governments are careful about how red mud is used because it can contain harmful substances like arsenic and chromium.
Ajka Alumina Plant Accident
On October 4, 2010, there was a serious accident at an alumina plant in Hungary. A wall holding back a red mud reservoir broke. About 700,000 cubic meters of red mud and water, which was very basic (pH 12), spilled out. This flood went into a river valley and covered parts of nearby towns. Sadly, 10 people died, and over a hundred were hurt. It also caused a lot of pollution in lakes and rivers.
History of the Process
Before the Bayer process, other methods were used. In 1859, a French scientist named Henri Étienne Sainte-Claire Deville developed a way to make alumina. He heated bauxite with sodium carbonate, then used carbon dioxide to get aluminium hydroxide. This was called the Deville process.
The Hall–Héroult process for making aluminium metal was invented in 1886. This meant there was a big need for a good way to make alumina.
The Bayer process was invented in 1888 by Carl Josef Bayer. He was working in Russia and trying to find a better way to make alumina for the textile industry. He found that if he made aluminium hydroxide come out of a basic solution, it formed crystals that were easy to clean. This was much better than older methods.
Because the Bayer process worked so well, it quickly replaced older methods. It was a big step forward in the field of hydrometallurgy, which is about using water solutions to get metals.
Over the years, engineers have made the Bayer process even better. Starting in 1967, improvements were made in Germany and Czechoslovakia. They found ways to save heat and use bigger machines. They used special equipment like heat exchangers to reuse energy. All these changes made the process more efficient and cheaper.
Today, the Bayer process is used to make almost all the world's alumina. This alumina is then used to produce the aluminium metal we use every day.
See also
- Ajka alumina plant accident
- Deville process
- Hall–Héroult process
- History of aluminium