History of aluminium facts for kids
Aluminium (or aluminum) is a metal that was once very rare and hard to get. For a long time, people didn't even know it existed. However, a compound called alum has been known since ancient times (around 5th century BCE). People used alum a lot for dyeing fabrics. During the Middle Ages, alum became a valuable product traded all over the world because it was so important for dyeing.
Later, scientists in the Renaissance thought alum was a salt of a new type of "earth." During the Age of Enlightenment, they figured out that this "earth," called alumina, was actually an oxide of a brand new metal. The discovery of this metal was announced in 1825 by a Danish scientist named Hans Christian Ørsted. His work was later continued by a German chemist named Friedrich Wöhler.
Because aluminium was so hard to make, it was very expensive, even more costly than gold! Its price only dropped after the first industrial production started in 1856 by French chemist Henri Étienne Sainte-Claire Deville. Aluminium became much more common thanks to the Hall–Héroult process, developed independently by French engineer Paul Héroult and American engineer Charles Martin Hall in 1886. Another important step was the Bayer process, created by Austrian chemist Carl Joseph Bayer in 1889. These methods are still used today to produce aluminium.
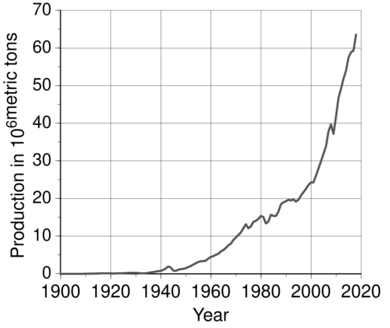
These new ways of making a lot of aluminium led to its widespread use. This light, rust-resistant metal became important in many industries and in our daily lives. Aluminium started being used in building and engineering. During World War I and World War II, aluminium was a super important material for making airplanes. Global production of aluminium jumped from 6,800 metric tons in 1900 to 2,810,000 metric tons in 1954. At that point, aluminium became the most produced non-ferrous metal, meaning it was made more than any metal except iron, even more than copper.
In the second half of the 20th century, aluminium became popular for transportation and packaging. People also started to worry about how aluminium production affected the environment, so aluminium recycling became more common. The metal started to be traded on markets in the 1970s. Production began to move from richer countries to developing ones. By 2010, China produced and used a huge amount of the world's aluminium. Global production kept rising, reaching 58,500,000 metric tons in 2015. Today, more aluminium is produced than all other non-ferrous metals combined.
Contents
The Early Story of Aluminium

The story of aluminium really begins with its compound, alum. The first time alum was written about was in the 5th century BCE by the Greek historian Herodotus. Ancient people used alum for many things. It helped dyes stick to fabric, was used in medicine, and even helped protect wood from fire. But the metal aluminium itself was unknown.
A Roman writer named Petronius wrote a story in his novel Satyricon about a special glass. This glass was shown to the emperor. When it was thrown on the ground, it didn't break, it just bent. The inventor then used a hammer to make it go back to its original shape. The emperor, worried that this new material would make gold less valuable, had the inventor killed. Some people think this special glass might have been an early form of aluminium. It's also possible that aluminium-containing alloys were made in China during the first Jin dynasty (266–420).
After the Crusades, alum became a very important product for international trade. It was essential for Europe's fabric industry. Most alum came from the Middle East, though there were small mines in Catholic Europe. This trade continued across the Mediterranean Sea until the mid-1400s. Then, the Ottomans greatly increased taxes on alum exports.
Just a few years later, a large amount of alum was found in Italy. Pope Pius II then stopped all imports from the East. He used the money from the new alum trade to start a war against the Ottomans. This new Italian alum was important in European medicine for a long time. However, the high prices set by the Pope eventually made other countries start their own production. Large-scale alum mining began in other parts of Europe in the 1500s.
Understanding What Alum Is
At the start of the Renaissance, scientists still didn't fully understand what alum was. Around 1530, a Swiss physician named Paracelsus realized that alum was different from other sulfates and suggested it was a salt of an "earth." In 1595, German chemist Andreas Libavius showed that alum was made from the same acid as other sulfates but with a different "earth." He suggested the name "alumina" for this undiscovered earth.
Many scientists, like German chemist Georg Ernst Stahl in 1702, mistakenly thought the unknown base of alum was similar to lime or chalk. This idea lasted for about 50 years. In 1722, German chemist Friedrich Hoffmann suggested that the base of alum was a unique earth. In 1728, French chemist Étienne Geoffroy Saint-Hilaire said alum was made from an unknown earth and sulfuric acid. He was wrong about some details, but his ideas helped others.
In 1754, German chemist Andreas Sigismund Marggraf successfully created the "earth of alum" by boiling clay in sulfuric acid and adding potash. He discovered that adding soda, potash, or an alkali to a solution of this new earth in sulfuric acid would create alum. He also found that this earth was alkaline and dissolved in acids when dried. Marggraf also described salts made from this earth, like chloride, nitrate, and acetate. In 1760, French chemist Théodore Baron d'Hénouville was confident that alumina was a metallic earth.
In 1767, Swedish chemist Torbern Bergman made alum by boiling alunite in sulfuric acid and adding potash. He also showed that alum was a "double salt," meaning it was made from two different salts. In 1776, Swedish-German chemist Carl Wilhelm Scheele proved that both alum and silica came from clay, but alum did not contain silicon. In 1782, French chemist Antoine Lavoisier thought alumina was an oxide of a metal. He believed this metal had such a strong attraction to oxygen that no known methods could separate them.
In 1815, Swedish chemist Jöns Jacob Berzelius suggested the formula AlO3 for alumina. The correct formula, Al2O3, was found by German chemist Eilhard Mitscherlich in 1821. This helped Berzelius figure out the correct atomic weight of the metal, which is 27.
Finding the Metal Itself

In 1760, Baron de Hénouville tried to turn alumina into metal but failed. He likely mixed alum with carbon and heated it. Other chemists tried similar experiments, even at higher temperatures, but only found metallic particles that were actually iron phosphide from impurities. Many scientists, including Lavoisier, tried to melt alumina with very hot flames, but they never found the metal.
In 1807, British chemist Humphry Davy used electricity to try and get metal from alumina. He succeeded in making an alloy, but it contained potassium and sodium, and he couldn't separate the aluminium. He also tried heating alumina with potassium, but again, no pure aluminium. Davy suggested naming the metal alumium in 1808 and aluminum in 1812, which led to the modern name. Other scientists preferred aluminium, but aluminum later became common in the United States.
In 1824, Danish physicist Hans Christian Ørsted tried to make the metal. He mixed dry aluminium chloride with potassium amalgam. He got a lump of metal that looked like tin. He showed his results and a sample of the new metal in 1825. He later wrote that "aluminium has a metallic luster and somewhat grayish color and breaks down water very slowly." This suggests he had an aluminium–potassium alloy, not pure aluminium. Ørsted didn't think his discovery was very important and didn't tell other famous chemists. Because of this, he is often not given credit for discovering the element.

In 1827, German chemist Friedrich Wöhler visited Ørsted and got permission to continue the aluminium research. Wöhler tried Ørsted's experiments but didn't find any aluminium. He then did a similar experiment, mixing dry aluminium chloride with potassium, and produced a powder of aluminium. Wöhler continued his research and in 1845, he was able to make small pieces of the metal and described some of its properties. His description shows that his aluminium was not completely pure.
For many years, Wöhler was credited as the discoverer because other scientists couldn't repeat Ørsted's experiment. However, in 1921, a Danish chemist named Johan Fogh found out why. Ørsted's experiment worked because he used a lot of extra aluminium chloride and an amalgam with low potassium content. In 1936, scientists from the American aluminium company Alcoa successfully recreated Ørsted's experiment. Still, many sources today credit Wöhler with discovering and successfully isolating aluminium in a purer form.
Early Ways to Make Aluminium in Factories
Wöhler's method couldn't make much aluminium, so the metal stayed rare and expensive. In 1852, aluminium cost $34 per ounce, while gold was $19 per ounce.
In 1854, French chemist Henri Étienne Sainte-Claire Deville announced a new way to produce aluminium in large amounts. He found that aluminium chloride could be reduced by sodium, which was easier and cheaper to use than potassium. Deville managed to produce a block of the metal. Napoleon III of France was very interested in aluminium for military use. He wanted to make weapons, helmets, and armor from this new light, shiny metal. Napoleon promised Deville unlimited money for his research. It's even said that Napoleon hosted a fancy dinner where the most important guests ate with aluminium utensils, while others used gold ones.
In 1855, twelve small blocks of aluminium were shown to the public for the first time at the World's Fair in Paris. The metal was called "the silver from clay" because it looked so much like silver. People were very excited, suggesting aluminium could be used in art, medicine, cooking, and tableware. Famous writers like Charles Dickens and Jules Verne imagined its future uses. However, some newspapers were less impressed, saying the amount of metal shown (about a kilogram) was not much for a discovery that was supposed to "turn the world upside down." Still, the fair led to the metal being sold commercially. That year, aluminium was sold for 300 French francs per kilogram. At the next fair in Paris in 1867, visitors saw aluminium wire and foil, as well as a new alloy called aluminium bronze, which was cheap to make, resisted rust, and had good properties.
At first, manufacturers didn't want to spend money on a new metal when they were already making popular metals like iron and bronze. Also, the aluminium produced wasn't very pure and its properties varied. Despite this, Deville and his partners opened the world's first industrial aluminium factory in Rouen in 1856. The factory later moved to Salindres in 1857. At one point, they produced 2 kilograms of 98% pure aluminium per day. In 1858, Deville started using bauxite as a source for alumina, which became known as the Deville process. By 1860, Deville sold his aluminium business to Henri Merle, whose company later controlled the French aluminium market.

Some chemists tried to use cryolite as a source, but with little success. British engineer William Gerhard opened a plant using cryolite in London in 1856, but it closed after three years due to problems. British ironmaster Isaac Lowthian Bell produced aluminium from 1860 to 1874. Deville's production grew to 1 metric ton per year in 1860 and 1.8 metric tons in 1872. However, demand for aluminium was low; for example, Deville's British agents sold only 15 kilograms in 1872. Aluminium was often compared to silver and used for jewelry and art. Its price steadily dropped from 240 French francs in 1859 to 120 francs in 1867.
More production sites appeared in the 1880s. British engineer James Fern Webster started industrial production of aluminium using sodium in 1882. His aluminium was purer than Deville's. In 1884, global aluminium production was 3.6 metric tons. That year, American architect William Frishmuth combined the production of sodium, alumina, and aluminium into one process, which lowered the cost to about $16 per pound. In 1886, American engineer Hamilton Castner found a cheaper way to make sodium, which further dropped the cost of aluminium production to $8 per pound. In 1889, German metallurgist Curt Netto launched a method using cryolite and sodium that produced aluminium with very few impurities.
Making Aluminium with Electricity

Aluminium was first made using electrolysis (using electricity to separate chemicals) in 1854 by German chemist Robert Wilhelm Bunsen and Deville. But their methods weren't good enough for factory production because electricity was hard to make efficiently back then. This changed with Belgian engineer Zénobe-Théophile Gramme's invention of the dynamo in 1870, which made it possible to create large amounts of electricity. The invention of three-phase current by Russian engineer Mikhail Dolivo-Dobrovolsky in 1889 allowed electricity to be sent over long distances.
Making a lot of aluminium using electrolysis was still tricky because the containers (electrolytic baths) couldn't handle the hot, melted salts for long. They would corrode. The first person to try to fix this for aluminium was American engineer Charles Bradley in 1883. He heated the aluminium salts from the inside, so the walls of the bath stayed cooler and the salts would solidify there, protecting the container. Bradley later sold his idea to the Cowles brothers, who used it to make aluminium alloys.
The first successful large-scale production method was developed independently by French engineer Paul Héroult and American engineer Charles Martin Hall in 1886. This is now known as the Hall–Héroult process. It's hard to use electrolysis on pure alumina because it has a very high melting point. Both Héroult and Hall realized that adding melted cryolite could greatly lower this melting point. Héroult got a patent in France in April and then in other European countries. He also applied for a U.S. patent. At first, Héroult couldn't find anyone interested in his invention. People told him there wasn't much demand for aluminium, only for aluminium bronze.
In 1888, Héroult and his partners founded Aluminium Industrie Aktiengesellschaft and started making aluminium bronze. Then, another company, Société électrométallurgique française, was founded in Paris. They convinced Héroult to come back to France, bought his patents, and made him director of a factory that soon produced pure aluminium.
At the same time, Hall was making aluminium using the same process at his home in Oberlin. He applied for a patent in July, and the patent office told him there was a conflict with Héroult's application. Hall later co-founded the Pittsburgh Reduction Company in 1888 and started producing aluminium. Hall's patent was granted in 1889. By September 1889, Hall's company was producing 385 pounds of aluminium at a cost of $0.65 per pound.
The Hall–Héroult process turns alumina into metal. Austrian chemist Carl Josef Bayer found a way to clean bauxite (the ore) to get pure alumina in 1888. This is now known as the Bayer process. Bayer heated bauxite with an alkali and washed it with water. After stirring the solution and adding a seed crystal, he got a pure precipitate of aluminium hydroxide, which turned into alumina when heated. In 1892, he discovered that the aluminium in bauxite dissolved in the leftover alkaline liquid after separating the solids. This was very important for using this method in factories.
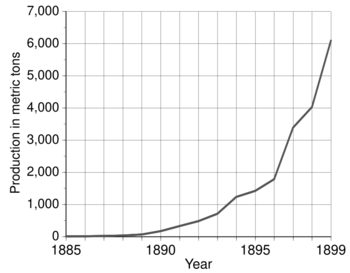
From 1856 to 1889, only 200 metric tons of unalloyed aluminium were produced using Deville's chemical method. But in 1890 alone, 175 metric tons were produced using the new methods! Production grew to 715 metric tons in 1893 and 4,034 metric tons in 1898. The price dropped to $2 per pound in 1889 and to $0.5 per pound in 1894.
By the end of 1889, aluminium made by electrolysis was consistently very pure. In 1890, Webster's factory became outdated when an electrolysis factory opened in England. Netto's company, whose main advantage was pure aluminium, closed the next year because electrolytic aluminium was just as good. Even Compagnie d'Alais et de la Camargue decided to switch to electrolytic production, opening their first plant using this method in 1895.
Modern aluminium production still uses the Bayer and Hall–Héroult processes. A big improvement came in 1920 when a team led by Swedish chemist Carl Wilhelm Söderberg introduced continuous electrodes. These new electrodes, made from a coke and tar paste, greatly increased the world's output of aluminium.
Aluminium Becomes Common
As prices for aluminium dropped, it became widely used in jewelry, eyeglass frames, and many everyday items by the early 1890s. Aluminium cookware started to be made in the late 1800s and slowly replaced copper and cast iron cookware in the early 1900s. Aluminium foil also became popular. Aluminium is soft and light, but scientists soon found that mixing it with other metals could make it harder while keeping it light. These aluminium alloys were used in many ways. For example, aluminium bronze was used for flexible bands, sheets, and wires, and became important in shipbuilding and aviation. A new aluminium alloy called duralumin, invented in 1903, was used in airplanes.
Aluminium recycling began in the early 1900s and became very common because aluminium can be recycled many times without losing its quality. At first, only unused metal from factories was recycled. During World War I, governments needed huge amounts of aluminium for light, strong airplane frames. They often helped pay for factories and the necessary electrical systems. Global aluminium production soared during the war: from 6,800 metric tons in 1900 to over 100,000 metric tons annually in 1916. The war created such a high demand that recycling also grew a lot. After the war, production dropped but then quickly grew again.

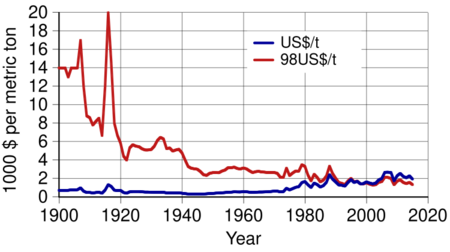
In the first half of the 20th century, the actual price of aluminium kept falling, from $14,000 per metric ton in 1900 to $2,340 in 1948 (in 1998 dollars). There were some exceptions, like a sharp price increase during World War I. Aluminium became plentiful. In 1919, Germany even started replacing its silver coins with aluminium ones. By the mid-20th century, aluminium was a part of everyday life, essential for housewares. Aluminium freight cars first appeared in 1931. Their lighter weight allowed them to carry more cargo. In the 1930s, aluminium became a material for civil engineering, used in buildings and their interiors. Its use in military engineering for airplanes and tank engines also increased.
Recycled aluminium was once thought to be not as good as new aluminium because it was harder to control its chemistry and remove waste. Recycling grew, but it depended on how much new aluminium was made. For example, when electricity prices dropped in the United States in the late 1930s, more new aluminium could be produced, making recycling less necessary, so recycling rates went down. By 1940, large-scale recycling of aluminium from used products began.

During World War II, production peaked again, exceeding 1,000,000 metric tons for the first time in 1941. Aluminium was used heavily in aircraft production and was extremely important for the war. In 1939, Germany was the world's top producer of aluminium, seeing it as an advantage in the war. Aluminium coins continued to be used, but by 1941, they were taken out of circulation to save the metal for military needs. After the United Kingdom was attacked in 1940, it started a big aluminium recycling program. The Minister of Aircraft Production asked the public to donate any household aluminium for building airplanes. The Soviet Union received 328,100 metric tons of aluminium from its allies from 1941 to 1945. This aluminium was used in aircraft and tank engines. Without these shipments, the Soviet aircraft industry's output would have dropped by more than half.
After the wartime peak, global production fell for three years but then quickly grew again. In 1954, the world produced 2,810,000 metric tons. This production was more than copper, making aluminium the most produced non-ferrous metal.
The Aluminium Age
Sputnik 1, Earth's first artificial satellite launched in 1957, was made of two joined aluminium hemispheres. All spacecraft since then have used aluminium. The aluminium can was first made in 1956 and used for drinks in 1958. In the 1960s, aluminium was used for wires and cables. Since the 1970s, high-speed trains have commonly used aluminium because it is strong for its weight. For the same reason, cars are using more and more aluminium.
By 1955, six major companies dominated the world market: Alcoa, Alcan, Reynolds, Kaiser, Pechiney, and Alusuisse. Together, they controlled 86% of the market. From 1945, aluminium use grew by almost 10% each year for nearly 30 years. It became more common in buildings, electric cables, foils, and the aircraft industry. In the early 1970s, the development of aluminium beverage cans gave it another boost. The real price of aluminium kept falling until the early 1970s, reaching $2,130 per metric ton in 1973 (in 1998 dollars). This price drop was mainly due to lower costs for getting and processing the metal, new technology, and increased production, which first went over 10,000,000 metric tons in 1971.
In the late 1960s, governments became aware of waste from industrial production. They created rules that encouraged recycling and proper waste disposal. Söderberg anodes, which were cheaper to use but more harmful to the environment, became less popular. Production started to shift back to older, pre-baked anodes. The aluminium industry began promoting the recycling of aluminium cans to avoid new restrictions. This led to a big increase in recycling of aluminium from used products. For example, in the United States, recycling of such aluminium increased 3.5 times from 1970 to 1980 and 7.5 times by 1990. Production costs for new aluminium also rose in the 1970s and 1980s, which also helped increase aluminium recycling. Better control over the metal's composition and improved refining technology made the quality difference between new and recycled aluminium smaller.
In the 1970s, the higher demand for aluminium made it a product that could be traded on markets. It joined the London Metal Exchange, the world's oldest industrial metal exchange, in 1978. Since then, aluminium has been traded for United States dollars, and its price changes with the dollar's exchange rate. The need to use lower-quality deposits, rising costs for energy and bauxite, and changes in exchange rates and greenhouse gas rules all increased the cost of aluminium. The real price grew in the 1970s.
The increase in real price and changes in taxes began to shift where aluminium was produced around the world. In 1972, the United States, the Soviet Union, and Japan produced almost 60% of the world's new aluminium. But by 2012, their combined share was only a little over 10%. Production started moving in the 1970s from these countries to Australia, Canada, the Middle East, Russia, and China. It was cheaper to produce there because of lower electricity prices and helpful government rules, like low taxes or subsidies. Production costs in the 1980s and 1990s went down because of new technology, lower energy and alumina prices, and high exchange rates for the United States dollar.
In the 2000s, the combined share of the BRIC countries (Brazil, Russia, India, and China) grew a lot in both production and use of new aluminium. China especially gained a huge share of world production due to many resources, cheap energy, and government support. China's share of consumption also increased from 2% in 1972 to 40% in 2010. The only other country with a double-digit percentage was the United States with 11%. In the United States, Western Europe, and Japan, most aluminium was used in transportation, engineering, construction, and packaging.
In the mid-2000s, rising prices for energy, alumina, and carbon (used in anodes) caused production costs to increase. This was made worse by changes in currency exchange rates, including a weaker United States dollar and a stronger Chinese yuan. The yuan's strength became important because most Chinese aluminium was relatively cheap.
Global output continued to grow. In 2018, it reached a record 63,600,000 metric tons before dropping slightly in 2019. More aluminium is produced than all other non-ferrous metals combined. Its real price (in 1998 United States dollars) in 2019 was $1,400 per metric ton ($2,190 per ton in today's dollars).
See also
- List of countries by primary aluminium production
 | Selma Burke |
 | Pauline Powell Burns |
 | Frederick J. Brown |
 | Robert Blackburn |