Lime (material) facts for kids

Lime is a general name for different natural minerals and materials that come from them. These materials are mostly made of calcium compounds like carbonates, oxides, and hydroxides. Think of it as a family of materials all related to calcium!
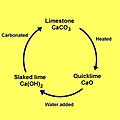
These lime materials are used a lot in building things, like making concrete and mortar. They are also important in many chemical processes. The rocks and minerals that lime comes from, mainly limestone and chalk, are mostly made of calcium carbonate. When limestone is heated very strongly (a process called "burning" or calcination), it changes into a material called quicklime (calcium oxide). Quicklime is very strong and can cause burns if not handled carefully. If you add water to quicklime, it changes again into slaked lime (calcium hydroxide), which is less strong but still very alkaline.
When people talk about "lime" in farming, they usually mean Agricultural lime. This type of lime helps improve soil for growing crops. In other cases, "lime" most often means slaked lime, because the more dangerous form, quicklime, is usually called by its specific name.
Contents
What is Lime?
Lime is not just one single thing. It's a group of materials that all contain the element calcium. The most common forms you'll hear about are:
- Limestone (calcium carbonate): This is a natural rock found in the ground. It's the starting point for making other types of lime.
- Quicklime (calcium oxide): This is a white, powdery material made by heating limestone. It's very reactive.
- Slaked lime (calcium hydroxide): This is made by adding water to quicklime. It's often used in building and other industries.
These different forms of lime have unique properties and uses, but they are all connected through calcium.
How is Lime Made?
Making lime involves a special process that changes limestone into other forms.
From Rock to Powder: Calcination
The first step is to take natural limestone or chalk. These rocks are mostly calcium carbonate. To make quicklime, the limestone is heated to very high temperatures, usually over 900°C (1650°F), in a special oven called a kiln. This heating process is called calcination.
During calcination, the heat causes the calcium carbonate in the limestone to break down. It releases carbon dioxide gas into the air. What's left behind is calcium oxide, which is known as quicklime. Quicklime is a white, powdery material. It's very reactive and can get hot when it touches water, so it needs to be handled with care.
Adding Water: Slaking
Once you have quicklime, you can add water to it. This process is called "slaking." When water is added to quicklime (calcium oxide), a chemical reaction happens. This reaction creates calcium hydroxide, which is known as slaked lime. Slaked lime is less reactive than quicklime and is often used as a paste or a powder.
This whole process, from limestone to quicklime and then to slaked lime, is sometimes called the "lime cycle."
Uses of Lime
Lime has been used by humans for thousands of years! It's a very versatile material.
In Building and Construction
One of the oldest and most important uses of lime is in building.
- Mortar and Plaster: Slaked lime is a key ingredient in mortar, which is like the "glue" that holds bricks and stones together in walls. It's also used in plaster to make smooth surfaces on walls and ceilings.
- Concrete: While cement is the main binder in modern concrete, lime can also be used in some concrete mixes.
- Roads: Lime can be added to soil to make it stronger and more stable, which is helpful when building roads and foundations.
In Agriculture
Farmers use Agricultural lime to improve their soil.
- Soil Health: Lime helps to balance the pH level of acidic soil, making it less sour. This allows crops to grow better and absorb nutrients more easily.
- Nutrients: It also adds important calcium to the soil, which plants need to be healthy.
Other Important Uses
Lime is also used in many other industries:
- Water Treatment: It helps clean drinking water and wastewater by removing impurities.
- Steel Making: In the steel industry, lime helps remove unwanted substances from molten iron.
- Paper Production: It's used in the process of making paper.
- Chemical Industry: Lime is a basic chemical that is used to make many other chemicals.
Images for kids
See also
 In Spanish: Cal (material) para niños
In Spanish: Cal (material) para niños
 | Anna J. Cooper |
 | Mary McLeod Bethune |
 | Lillie Mae Bradford |