Hall–Héroult process facts for kids
The Hall–Héroult process is a super important way to make aluminium. It's how almost all the new aluminum we use today is produced! This process takes aluminium oxide (called alumina), which comes from a rock called bauxite. It dissolves the alumina in a special melted salt called cryolite. Then, it uses electricity to pull the pure aluminum out.
This process happens at very high temperatures, around 940–980 °C (1700 to 1800°F). It makes aluminum that is 99.5–99.8% pure. Making aluminum this way uses a lot of electrical energy. It also creates some carbon dioxide and other gases, which can cause air pollution and affect our climate.
Contents
How Aluminium is Made
Why Making Aluminium is Tricky
Making pure aluminum is not easy. You can't just use electricity on aluminum salt in water. This is because water would react with the aluminum. Also, aluminium oxide (alumina) melts at a super high temperature, over 2000 °C (3700°F). It would be too hard and expensive to melt it just to use electricity.
The Hall–Héroult process solves this problem. It dissolves alumina (Al2O3) in melted cryolite (Na3AlF6). This mix melts at a much lower temperature, around 1000 °C (1832°F). This makes it possible to use electricity to get the aluminum.
The Science Behind It
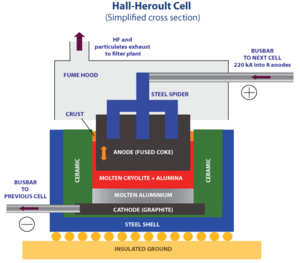
In the Hall–Héroult process, electricity flows through the melted mix. This causes chemical reactions at two special parts called electrodes.
At the negative electrode (the cathode):
This means aluminum parts (ions) grab electrons and turn into pure liquid aluminum.
At the positive electrode (the anode):
Here, oxygen from the alumina combines with carbon from the anode. This creates carbon dioxide gas.
So, the overall main reaction is:
This shows that alumina and carbon turn into aluminum and carbon dioxide.
Cryolite is used because it melts at a lower temperature when alumina is added. It also dissolves alumina well and helps electricity flow through the mix. Plus, it's lighter than liquid aluminum, so the aluminum sinks to the bottom.
Sometimes, other things like aluminium fluoride (AlF3) are added. This helps lower the melting point even more, so the process can happen between 940 and 980 °C.
How the Cells Work
Factories run these aluminum-making cells 24 hours a day. This keeps the materials inside melted. The heat from the electricity flowing through the cell helps keep it hot.
The liquid aluminum is heavier than the melted cryolite. So, the aluminum sinks to the bottom of the cell. Workers collect this liquid aluminum every 1 to 3 days using a special tube called a siphon. More alumina is added to the cells as aluminum is taken out.
The cells also produce gases at the anode. Most of this gas is carbon dioxide. There's also some hydrogen fluoride (HF) from the cryolite. Modern factories try to capture and reuse these fluoride gases.
Sometimes, the melted material inside the cell is stirred. This can help make aluminum faster. However, it can also make the aluminum less pure.
The Electrodes Used
The electrodes in these cells are mostly made from a type of carbon called coke. A sticky material called pitch is used to hold the coke together. These materials must be very pure so they don't add unwanted stuff to the aluminum.
There are two main types of anodes (positive electrodes) used in the Hall–Héroult process:
Söderberg Anodes
In cells with Söderberg anodes, there's only one large anode per cell. As the bottom of the anode is used up, new material (coke and pitch) is added to the top. The heat from the process bakes this new material into a solid carbon anode.
However, making aluminum with Söderberg anodes releases more harmful gases into the air. Because of this, the other type of anode has become more common.
Prebaked Anodes
Prebaked anodes are made and baked in huge ovens before they are put into the cells. Each cell usually has about 24 prebaked anodes arranged in rows. A computer system slowly lowers each anode as its bottom part gets used up.
Prebaked anodes can be placed closer to the liquid aluminum. This makes the process more efficient. They also cause fewer problems with something called the "anode effect" (explained below). However, cells using prebaked anodes are more expensive to build and need more work to replace the used anodes.
The inside of the cell, which acts as the negative electrode (cathode), is also made from coke and pitch. Cathodes wear out much slower than anodes. They usually need to be replaced only every 2 to 6 years. When this happens, the whole cell has to be shut down.
What is the Anode Effect?
The anode effect happens when too many gas bubbles form at the bottom of the anode and stick together. This creates a layer of gas that stops the electrolyte from touching the anode properly.
When this happens, the electricity has to go through a smaller area. This makes those areas get very hot, which makes the gas layer expand even more. The anode effect makes the cell less efficient and produces less aluminum. It also creates more harmful gases like tetrafluoromethane (CF4) and hexafluoroethane (C2F6). These are strong greenhouse gases, which contribute to climate change. The anode effect is mostly a problem with Söderberg anodes.
History of Aluminium Production
Aluminium: Once a Rare Metal
Aluminium is one of the most common metals on Earth. But it's almost never found as a pure metal. It's usually mixed with other elements in minerals like bauxite.
Before the Hall–Héroult process, making pure aluminum was very difficult and expensive. People had to heat aluminum ore with other expensive metals like sodium or potassium in a vacuum. This made aluminum more costly than even gold or platinum!
In 1855, at a big exhibition in France, bars of aluminum were shown next to the French crown jewels. It's said that Emperor Napoleon III of France saved his few aluminum dinner plates for his most important guests. Even when the tip of the Washington Monument was made of aluminum, it was still more expensive than silver.
Two Young Inventors
The Hall–Héroult process was discovered independently and almost at the same time in 1886. Two 22-year-old men, Charles Martin Hall from the United States and Paul Héroult from France, both figured it out.
In 1888, Charles Martin Hall opened the first big aluminum factory in Pittsburgh. This company later became known as Alcoa. In 1997, the Hall–Héroult process was recognized as a very important chemical discovery in American history.
Making Aluminium Affordable
Thanks to the Hall–Héroult process and cheaper electric power, aluminum became much less expensive. It changed from being a precious metal to an everyday material.
This made it possible to use aluminum for many new things. For example, people like Hugo Junkers could build thousands of metal airplanes. Howard Lund could make aluminum fishing boats.
However, making aluminum still has an environmental cost. In 2012, it was estimated that making one ton of aluminum created about 12.7 tons of carbon dioxide emissions.
See also
- Bayer process
- History of aluminium
- Solid oxide Hall–Héroult process
- Hoopes process
- Downs cell
 | DeHart Hubbard |
 | Wilma Rudolph |
 | Jesse Owens |
 | Jackie Joyner-Kersee |
 | Major Taylor |