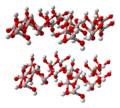Gibbsite facts for kids
Quick facts for kids Gibbsite |
|
|---|---|

Gibbsite from Brazil
|
|
| General | |
| Category | Hydroxide minerals |
| Formula (repeating unit) |
Al(OH)3 |
| Strunz classification | 04.FE.10 |
Gibbsite is a common mineral that is a form of aluminium hydroxide. Its chemical formula is Al(OH)3. You might also hear it called hydrargillite. It is a very important source of aluminium, which is a metal used in many everyday things. Gibbsite is a main part of a rock called Bauxite, which is the world's most important aluminium ore.
Contents
What is Gibbsite?
Gibbsite is a mineral that forms naturally in the Earth. It is made up of aluminium, oxygen, and hydrogen atoms. These atoms are arranged in a special way to create its unique crystal structure.
Gibbsite is often found in places where rocks have been weathered by rain and chemicals over a long time. This process helps to create the conditions needed for gibbsite to form. It can look like small, flat crystals or a powdery substance.
How Gibbsite Forms
Gibbsite usually forms in tropical and subtropical areas. In these warm, wet places, rain and water slowly break down rocks that contain aluminium. This process is called weathering.
As the rocks break down, the aluminium in them combines with water to create aluminium hydroxide. Over time, this aluminium hydroxide can turn into gibbsite. It often forms in soil and clay deposits.
Why is Gibbsite Important?
Gibbsite is extremely important because it is a major source of aluminium. Aluminium is a lightweight and strong metal that we use for many things.
Aluminium Ore
An ore is a rock or mineral from which a valuable metal can be taken out. Gibbsite is one of the main minerals found in Bauxite, which is the main ore used to produce aluminium.
To get aluminium from gibbsite, the bauxite rock goes through a special process. First, the bauxite is crushed and mixed with chemicals to separate the gibbsite. Then, the gibbsite is heated to a very high temperature. This process removes the water and leaves behind pure aluminium oxide, also known as alumina.
Finally, the alumina is melted and an electric current is passed through it. This separates the aluminium metal from the oxygen. This whole process needs a lot of energy, but it gives us the aluminium we use every day.
Uses of Aluminium
Aluminium is a very useful metal because it is light, strong, and does not rust. Here are some ways we use aluminium:
- Airplanes: Because it is light and strong, aluminium is perfect for building aircraft.
- Drink Cans: Most soda and juice cans are made from aluminium. They are easy to recycle.
- Foil: Aluminium foil is used for cooking and wrapping food.
- Building Materials: It is used in window frames, doors, and other parts of buildings.
- Cars and Bikes: Aluminium helps make vehicles lighter, which saves fuel.
- Electronics: Many electronic devices, like laptops and phones, use aluminium parts.
Images for kids
-
Ball-and-stick model of the part of the crystal structure of gibbsite
See also
 In Spanish: Gibbsita para niños
In Spanish: Gibbsita para niños
 | Bayard Rustin |
 | Jeannette Carter |
 | Jeremiah A. Brown |