Water purification facts for kids
Water purification is the process of making water clean and safe to use. This usually means removing unwanted chemicals, tiny living things like bacteria, and other particles from the water. The main goal is to make water safe for people to drink. But water can also be purified for other uses, like in hospitals, for making medicines, or in factories.
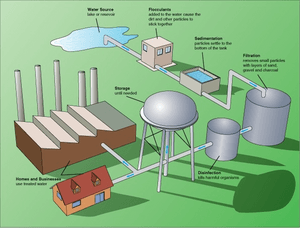
There are many ways to clean water. Some methods are physical, like filtering or letting things settle down (called sedimentation). Other methods are biological, using special slow sand filters or activated carbon. Chemical methods use substances like flocculation chemicals or chlorine. Sometimes, even ultraviolet light is used.
Water purification helps to get rid of tiny particles, parasites, bacteria, algae, viruses, and fungi. It also reduces the amount of dissolved stuff in the water.
Governments set rules for how clean water quality must be for drinking. These rules say what chemicals and tiny living things can be in the water. Many countries also require a certain amount of disinfectant (like chlorine) to stay in the water after it leaves the cleaning plant. This helps keep the water safe as it travels through pipes to homes.
You can't tell if water is safe to drink just by looking at it. Simple things like boiling water or using a home activated carbon filter might not remove all harmful things, especially if you don't know where the water came from. To really know what's in water and how to clean it, you need special chemical tests.
In 2007, the World Health Organization (WHO) reported that over a billion people didn't have access to safe drinking water. A lot of sickness, especially diarrheal disease, is caused by unsafe water and poor hygiene. Sadly, 1.8 million people die from diarrheal disease every year. The WHO believes that most of these deaths could be stopped by having access to safe water. Simple ways to clean water at home, like using chlorine, filters, or sunlight, and storing it safely, could save many lives. Stopping waterborne diseases is a big goal for public health, especially in developing countries.
Where Does Our Water Come From?
Water for purification comes from different places. Each source has its own challenges for cleaning.
Groundwater
Some deep groundwater can be very old, having fallen as rain thousands or even millions of years ago. Soil and rock layers naturally filter this water very well before it's pumped out. This water can come out as artesian springs or be pulled from wells.
Deep groundwater usually has very few harmful bacteria or protozoa. However, it often has many dissolved minerals, like carbonates and sulfates of calcium and magnesium. Sometimes, it also contains chloride and bicarbonate. We might need to remove iron or manganese from this water to make it taste better and be good for cooking or washing clothes. Sometimes, it also needs disinfection.
Upland Lakes and Reservoirs
These are usually found high up in mountains, at the start of river systems. They are often far from towns, with special protected areas around them to prevent pollution. They usually have low levels of bacteria and other harmful things, but some protozoa or algae might be present. If the land around is forested or peaty, the water can be colored by humic acids. Many of these sources also have a low pH, which needs to be adjusted.
Rivers, Canals, and Lowland Reservoirs
Water from rivers, canals, and lowland reservoirs often has a lot of bacteria. It can also contain algae, many floating particles, and various dissolved substances. This water usually needs more cleaning than groundwater or upland water.
New Ways to Get Water
- Water from Air: Atmospheric water generation is a new way to get clean drinking water. It works by cooling the air to collect water vapor.
- Rain and Fog: Rainwater harvesting or fog collection gathers water from the air. This is very useful in places with long dry seasons or where there is a lot of fog but little rain.
- Taking Salt Out of Seawater: Desalination is the process of removing salt from seawater to make it drinkable.
How Water is Cleaned
The main goal of water treatment is to remove unwanted things from the water and make it safe to drink or suitable for other uses. The way water is cleaned depends on how dirty the water is, how much it costs to clean it, and how clean the water needs to be at the end.
Here are the common steps used in water purification plants. Not all steps are used in every plant; it depends on the size of the plant and the quality of the water they start with.
First Steps (Pre-treatment)
- Pumping and Holding: Most water needs to be pumped from its source into pipes or large holding tanks. The pipes and tanks must be made of materials that won't add new dirt to the water.
- Screening: The very first step for surface water (like from rivers) is to remove large items. This includes sticks, leaves, trash, and other big pieces that could mess up the cleaning process. Deep groundwater usually doesn't need this step.
- Storage: River water can be stored in large reservoirs for days or even months. This allows some natural cleaning to happen. It also helps ensure there's enough water during dry times or if the river gets polluted for a short period.
- Adjusting pH: Water can be acidic or alkaline. If the water is too acidic (pH lower than 7), substances like lime or soda ash are added to make it less acidic.
Making Water Clear (Flocculation)
Flocculation is a process that makes the water clear by removing cloudiness or color. This is done by adding special chemicals called coagulants. These chemicals make tiny particles in the water stick together. At first, these particles are very small. But as the water is gently stirred, they join to form bigger, heavier clumps called "floc."
Many small particles that were in the dirty water attach to these floc clumps. This way, the floc collects most of the suspended dirt from the water. The floc is then removed, usually by passing the water through a filter made of sand or a mix of sand and anthracite coal.
Common chemicals used to create floc include:
- Iron (III) hydroxide
- Aluminium hydroxide
- Aluminium hydroxychloride
Letting Things Settle (Sedimentation)
After flocculation, the water goes into a large tank called a sedimentation basin or clarifier. The water flows very slowly here, giving the heavy floc clumps time to sink to the bottom. This tank usually holds water for at least four hours. As the large particles sink, they also help to pull down smaller particles with them.
A layer of sludge forms at the bottom of the tank from the settled particles. This sludge must be removed and treated. Some tanks have machines that constantly clean the bottom, while others need to be taken out of service for cleaning.
Filtering the Water
After most of the floc has settled, the water is filtered to remove any remaining floating particles and tiny floc pieces. The most common type of filter is a rapid sand filter. Water flows down through layers of sand. Often, there's a layer of activated carbon or anthracite coal on top of the sand. This top layer helps remove organic compounds that can make water taste or smell bad.
The spaces between sand particles are bigger than the smallest particles in the water. So, simple straining isn't enough. Most particles pass through the very top layers but get stuck deeper in the filter's pores or stick to the sand particles.
To clean the filter, water is pushed quickly upward through it, in the opposite direction of normal flow. This is called backflushing or backwashing. It removes the trapped particles. Sometimes, compressed air is blown up from the bottom first to break up the compacted filter material, which helps the backwashing process. This is called air scouring.
Filters have several advantages:
- They can remove much smaller particles than simple paper or sand filters.
- They filter out almost all particles larger than their tiny holes.
- They are thin, so water flows through them quickly.
- They are strong and can handle pressure.
- They can be cleaned and used again.
Membrane filters are also widely used. They have very tiny pores that can filter out almost all particles larger than 0.2 micrometers, including harmful things like Giardia and Cryptosporidium. However, no filter can remove substances that are truly dissolved in the water, like certain chemicals or heavy metals.
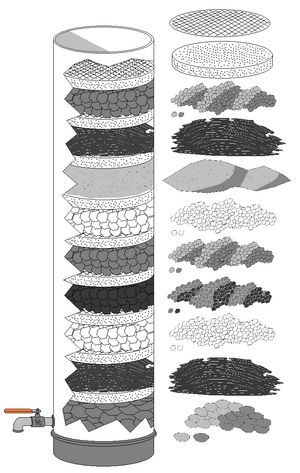
Slow Sand Filters
Slow sand filters can be used where there is a lot of land available, as water must pass through them very slowly. These filters rely on living organisms, not just physical straining, to clean the water. They are built with careful layers of sand, with coarser sand and gravel at the bottom and the finest sand on top. Drains at the bottom collect the cleaned water.
The cleaning happens because a thin layer of living material, called the Schmutzdecke, grows on the surface of the filter. This layer traps and breaks down impurities. A good slow sand filter can work for many weeks or months if the water is pre-treated well. These filters are not backwashed. Instead, the top layer of sand is scraped off when the water flow slows down due to too much biological growth.
Lava Filters
Lava filters are similar to sand filters and also need a lot of space. Like sand filters, they use biological processes to clean water. But they are made of two layers of lava pebbles and a top layer of special soil. Water-purifying plants like irises are planted on top. These filters also have drains at the bottom to collect the clean water.
Removing Dissolved Substances
- Ultrafiltration: These filters use special plastic membranes with tiny holes. They can filter out dissolved substances without needing the chemicals used in flocculation.
- Ion Exchange: These systems use special resins to swap unwanted dissolved ions (like Ca2+ and Mg2+ that cause hard water) with harmless ions (like Na+). Ion exchange can also remove toxic ions like nitrate or mercury.
- Electrodeionization: Water passes between positive and negative electrodes. Special membranes allow only positive ions to move toward the negative electrode and only negative ions toward the positive electrode. This creates very pure water.
Other Cleaning Methods
Besides large-scale methods, there are smaller, more natural ways to clean water. These often use mechanical and biological processes.
- Mechanical Systems: These include sand filtration, lava filter systems, and systems that use UV light.
- Biological Systems:
* Plant Systems: Like constructed wetlands and treatment ponds, where plants help clean the water. * Compact Systems: Such as activated sludge systems and biofilters, which use tiny organisms to break down pollutants.
Often, several of these systems are combined in stages to clean the water thoroughly.
Killing Germs (Disinfection)
Disinfection is the final step in cleaning drinking water. It kills any harmful pathogens (germs) that might have passed through the filters.
- Chlorination: The most common way to disinfect water is by adding some form of chlorine, like chloramine or chlorine dioxide. Chlorine is a strong chemical that quickly kills many harmful germs.
- Ozone: Ozone (O3) is a powerful gas that is toxic to most waterborne organisms. It's widely used in Europe.
- UV Radiation: UV radiation (light) is very good at deactivating cysts (tough forms of some germs), as long as the water isn't too cloudy. The main downside is that, like ozone, it doesn't leave any lasting protection in the water.
- Hydrogen Peroxide: This is another disinfectant. It works similarly to ozone but is slower and can lower the water's pH.
Images for kids
-
Rainbow trout are used in water purification plants to detect sudden water pollution.
See also
 In Spanish: Purificación de agua potable para niños
In Spanish: Purificación de agua potable para niños