Sulfate facts for kids
Quick facts for kids Sulfate |
|
|---|---|
 |
|
| IUPAC name | Sulfate |
| Identifiers | |
| CAS number | |
| PubChem | |
| EC number | 233-334-2 |
| ChEBI | CHEBI:16189 |
| SMILES | S(=O)(=O)([O-])[O-] |
|
InChI
InChI=1/H2O4S/c1-5(2,3)4/h(H2,1,2,3,4)/p-2
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Conjugate acid | Hydrogen sulfate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
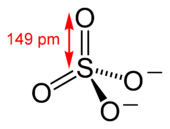
A sulfate is a special kind of ion made of several atoms. It has one sulfur atom and four oxygen atoms, written as SO2−
4. This ion has a negative electric charge.
Sulfates are very common in everyday life and are used a lot in different industries. They are basically salts that come from sulfuric acid. Many sulfates are made using this acid.
Contents
What are sulfates used for?
Sulfates are super useful in many industries. Here are some important ones:
- Gypsum: This is a natural mineral, a type of calcium sulfate. It's used to make plaster, which is important for building houses and other structures. About 100 million tons of gypsum are used each year!
- Copper sulfate: This is often used to stop algae from growing, especially in water. It's also used in special batteries called galvanic cells.
- Iron sulfate: You might find this in vitamin supplements for people and animals. It's also used to help plants grow in soil.
- Magnesium sulfate: You might know this as Epsom salts. People use it in baths for relaxation and muscle aches.
- Lead sulfate: This forms inside car batteries when they are used.
- Sodium Laureth Sulfate (SLES): This is a common ingredient in shampoos and soaps. It helps them make bubbles and clean things.
- Polyhalite: This is a natural mineral that contains potassium, calcium, and magnesium sulfates. It's used as a fertilizer to help crops grow.
Where do we find sulfates in nature?
Sulfates are also found naturally. Some tiny living things, called sulfate-reducing bacteria, live in places without much oxygen, like in mud or near deep-sea vents. These bacteria use sulfates to get energy.
How do sulfates affect our environment?
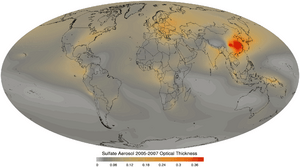
Sulfates can be found as tiny particles in the air, called aerosols. These particles come from burning things like fossil fuels (oil, coal, gas) and biomass (plants and animal waste).
When sulfates are in the air, they can make the atmosphere more acidic. This can lead to acid rain, which can harm plants, animals, and even buildings.
Some special bacteria, like Desulfovibrio desulfuricans, can actually help clean up buildings. They can remove the black sulfate crust that sometimes forms on old structures.
How sulfates affect Earth's climate
Sulfates have a big impact on our climate, mainly by reflecting sunlight. This helps to cool the Earth down. Think of it like a tiny mirror in the sky! This cooling effect helps to balance out some of the warming caused by greenhouse gases. This effect is strongest near big industrial areas.
Sulfates also affect clouds. They can act as tiny seeds for clouds, called cloud condensation nuclei. When there are more of these seeds, clouds form with many smaller water droplets. These smaller droplets are better at scattering sunlight, which also helps to cool the Earth.
Scientists are still studying all the ways sulfates affect clouds and our climate. It's a complex topic, but it's clear that sulfates play a role in global dimming, which is when less sunlight reaches the Earth's surface.
Sulfates are also a major part of the aerosol layer that forms high up in the atmosphere after big volcano eruptions, like the one from Mount Pinatubo in the Philippines in 1991. This layer can cool the climate for a year or two after an eruption.
See also
 In Spanish: Sulfato para niños
In Spanish: Sulfato para niños