Carbonate facts for kids
Quick facts for kids Carbonate |
|
|---|---|
 |
|
 |
|
 |
|
|
Preferred IUPAC name
Carbonate
|
|
|
Trioxidocarbonate
|
|
| Identifiers | |
| CAS number | |
| PubChem | |
| SMILES | C(=O)([O-])[O-] |
|
InChI
InChI=1/CH2O3/c2-1(3)4/h(H2,2,3,4)/p-2
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Conjugate acid | Bicarbonate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
A carbonate is a special type of chemical compound. It comes from carbonic acid (H
2CO
3). What makes a carbonate a carbonate is the carbonate ion. This is a polyatomic ion, meaning it's a group of atoms with an electrical charge. Its chemical formula is CO2−
3, which means it has one carbon atom and three oxygen atoms, with a negative charge of 2.
The word "carbonate" can also mean a process called carbonation. This is when you add carbon dioxide gas to water. It makes drinks fizzy, like soda! You can also do this by dissolving carbonate or bicarbonate salts in water.
In geology (the study of Earth's rocks), "carbonate" refers to certain carbonate minerals and carbonate rock. These rocks are mostly made of the carbonate ion. Carbonate minerals are very common in rocks formed from chemicals settling out of water.
Some common carbonate minerals include:
- Calcite (or calcium carbonate, CaCO
3). This is the main part of limestone. It also makes up mollusc shells and coral skeletons. - Dolomite (a calcium-magnesium carbonate, CaMg(CO
3)
2). - Siderite (or iron(II) carbonate, FeCO
3). This is an important source of iron.
People have used sodium carbonate ("soda") and potassium carbonate ("potash") for a long time. They are used for cleaning, preserving things, and making glass. Carbonates are also used in many industries. For example, they are used to make Portland cement and lime. They are also found in ceramic glazes.
Contents
What is the Structure of a Carbonate Ion?
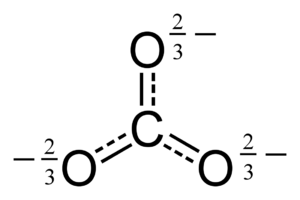
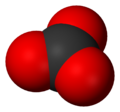
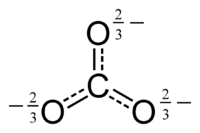
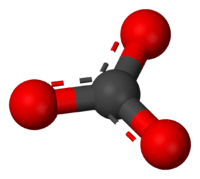
The carbonate ion is the simplest "oxocarbon anion." This means it's a negatively charged ion made of carbon and oxygen. It has one carbon atom in the middle. Three oxygen atoms surround it. They are arranged in a flat triangle shape. This shape is called "trigonal planar."
The carbonate ion has a total electrical charge of -2. It is related to the bicarbonate ion (HCO−
3). The bicarbonate ion is related to carbonic acid (H
2CO
3).
Scientists use something called a "Lewis structure" to show how atoms are connected. For the carbonate ion, a simple Lewis structure shows two single bonds to oxygen atoms with negative charges. It also shows one double bond to a neutral oxygen atom.
But this simple picture doesn't quite match what we observe. In reality, all three bonds in the carbonate ion are the same length. All three oxygen atoms are identical. This happens because of something called resonance. Resonance means the electrons are not stuck in one place. They are shared across all three oxygen atoms.
You can think of it like this: the electrons are "delocalized." This means they are spread out over the whole ion. This makes the bonds all equal and the charges spread out too.
What are the Chemical Properties of Carbonates?
When you heat most metal carbonates, they break down. They release carbon dioxide gas. What's left behind is an oxide of the metal. This process is called calcination. For example, heating calcium carbonate (limestone) makes calcium oxide (quicklime) and carbon dioxide:
- CaCO
3 → CaO + CO
2
Carbonate can also attach to many metal ions. It acts like a "ligand." This means it forms a bond with the metal.
Some carbonates dissolve easily in water. These include carbonates of lithium, sodium, potassium, rubidium, caesium, and ammonium. But carbonates of other metals, like calcium, often don't dissolve well in water.
Calcium carbonate (CaCO
3) is a good example of an insoluble carbonate. It can build up in pipes as "scale." This can block water flow. Hard water has a lot of this material. This is why we sometimes need to "soften" water.
If you add acid to carbonates, they usually release carbon dioxide gas.
- CaCO
3 + 2 HCl → CaCl
2 + CO
2 + H
2O
This is why you can use acid to remove limescale.
In water, carbonate, bicarbonate, carbon dioxide, and carbonic acid are always changing. They are in a dynamic balance. This balance changes depending on how acidic or basic the water is (its pH). It also changes with temperature and pressure.
For example, sodium carbonate is basic. Sodium bicarbonate is weakly basic. Carbon dioxide itself is a weak acid.
What are Organic Carbonates?
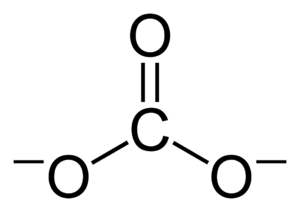
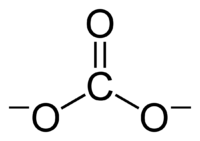
In organic chemistry, a carbonate can also be a part of a larger molecule. This is called a "functional group." It has a carbon atom connected to three oxygen atoms. One of these oxygen atoms is double-bonded to the carbon. These compounds are also known as "organocarbonates" or "carbonate esters." Their general formula is R–O–C(=O)–O–R′. Some important organic carbonates include dimethyl carbonate and ethylene carbonate.
How Carbonates Help Keep Things Balanced (Buffers)
Carbonates play a very important role in keeping things balanced, like the pH of your blood. Your blood needs to stay in a very specific pH range (around 7.37–7.43). Three reversible reactions involving carbonate, bicarbonate, and carbonic acid act as a buffer. A buffer helps to resist changes in pH.
Here's a simplified idea:
- When your body makes too much acid (more H+ ions), bicarbonate (HCO−
3) can react with it. This forms carbonic acid (H
2CO
3). This helps to remove the extra acid. - Carbonic acid can then turn into carbon dioxide gas (CO
2(g)) and water. - When you breathe out, you remove carbon dioxide. This helps to keep the balance.
This is an example of Le Châtelier's principle. This principle says that if you change the conditions of a system in balance, the system will try to adjust to reduce that change. So, if you remove carbon dioxide, the body will try to make more. This helps keep your blood pH stable.
A similar buffer system works in the oceans. This is very important for climate change and the long-term carbon cycle. Many sea creatures, especially coral, build their shells and skeletons from calcium carbonate. If the ocean gets warmer, carbonate dissolves more easily. This can make it harder for these creatures to build their homes. It also means more carbon dioxide can be released into the atmosphere, which can make Earth even warmer.
Where are Carbonates Found Outside Earth?
Scientists often think that finding carbonates in rocks is a strong sign that liquid water was once present. This is because carbonates usually form in water.
However, recent observations of a planetary nebula called NGC 6302 show signs of carbonates in space. It's unlikely that liquid water like Earth's exists there. This suggests that carbonates might form in other ways too.
Small amounts of carbonate deposits have also been found on Mars. This was done using special cameras that look at light reflected from the surface. Martian meteorites (rocks from Mars that landed on Earth) also have small amounts of carbonates. This suggests that groundwater might have existed in places like Gusev crater and Meridiani Planum on Mars.
Related pages
- Bicarbonate
- Calcium carbonate
- Polycarbonate
- Sodium carbonate
- Cap carbonates
- Oxalate
- Peroxocarbonate
- Sodium percarbonate
Images for kids
See also
 In Spanish: Carbonato para niños
In Spanish: Carbonato para niños