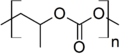Carbonate ester facts for kids
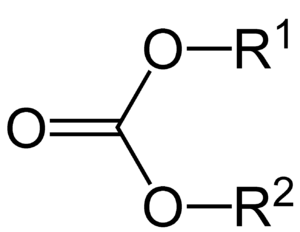
Carbonate esters, also known as organic carbonates, are special chemical compounds. They are a type of ester, which means they are made from an acid and an alcohol. Think of them as a building block in chemistry.
These compounds have a central carbon atom connected to three oxygen atoms. Two of these oxygen atoms are linked to other carbon chains. This special arrangement is called a "functional group." It helps us understand how these chemicals behave.
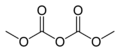
You might not know it, but carbonate esters are all around you! They are used to make plastics like polycarbonate, which is found in things like eyeglass lenses, compact discs, and even bulletproof glass. Smaller carbonate esters are also used as solvents, which are liquids that can dissolve other substances. For example, dimethyl carbonate can help other chemicals mix together.
Contents
What Are Carbonate Esters Used For?
Carbonate esters are very useful in many different ways. They are often used as solvents because they can dissolve many different substances.
In Batteries
One important use for organic carbonates is in lithium batteries. These are the batteries that power your phones, laptops, and many electric cars. Carbonate esters like dimethyl carbonate and ethylene carbonate are good at dissolving the lithium salts inside these batteries. This helps the batteries work well. Sometimes, different carbonate esters are mixed to make the battery work even better.
As Solvents in Chemistry
Carbonate esters are also used as solvents in making other chemicals. They are known as "polar solvents," which means they have a slight electrical charge. This helps them dissolve many different kinds of chemicals.
They can stay liquid over a very wide range of temperatures. For example, propylene carbonate stays liquid from a very cold −55 °C (which is -67 °F) all the way up to 240 °C (which is 464 °F)! This makes them useful for many different chemical reactions.
Another great thing about many carbonate esters is that they are not very harmful to the environment. They can also break down naturally over time, which is called "biodegradability."
As a Preservative
You might even find a carbonate ester in your drinks! Dimethyl dicarbonate is often used as a beverage preservative. This means it helps keep drinks fresh and safe to drink for longer. It can also be used to clean and sterilize drinks.
How Are Carbonate Esters Made?
Scientists and chemists make carbonate esters in a few different ways. They are not usually made from simple inorganic carbonate salts.
One common way to make them is by reacting an alcohol (or a similar chemical called a phenol) with a substance called phosgene. Another method involves reacting an alcohol with carbon monoxide and an oxidizer, which is a chemical that helps other chemicals combine with oxygen.
Sometimes, one type of carbonate ester can be changed into another type. This process is called "transesterification."
It's also possible to try and make carbonate esters directly from methanol and carbon dioxide. However, this reaction is a bit tricky because it doesn't naturally want to happen very easily. Scientists are working on ways to make this process more efficient, perhaps by using special membranes to remove water and help the reaction along.
Images for kids
-
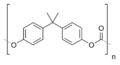
Poly(bisphenol A carbonate), a commercially important plastic (Lexan)
 | James B. Knighten |
 | Azellia White |
 | Willa Brown |