Solvay process facts for kids

The Solvay process is a clever way to make two important chemicals: sodium carbonate (also called soda ash) and sodium bicarbonate (baking soda). It uses common materials like salt, limestone, and ammonia. This process was invented by a Belgian chemist named Ernest Solvay in the 1860s. It changed how these chemicals were made, making them much cheaper and easier to get.
Contents
What is the Solvay Process?
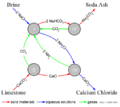
The Solvay process is a series of chemical reactions. It takes simple ingredients and turns them into useful products. The main idea is to mix carbon dioxide gas with a solution of ammonia and sodium chloride (common salt).
The Main Steps
The process happens in several stages. Here's a simplified look at how it works:
- First, salt water is mixed with ammonia. This creates a special solution.
- Next, carbon dioxide gas is bubbled through this solution. The carbon dioxide comes from heating limestone.
- When these mix, a solid substance called sodium bicarbonate forms. This is the same baking soda you might use at home!
- The sodium bicarbonate is then separated. It can be sold as baking soda.
- To make sodium carbonate, the sodium bicarbonate is heated. This heating process removes water and carbon dioxide, leaving behind sodium carbonate.
- An important part of the Solvay process is that it recycles most of the ammonia. This makes the process more efficient and less wasteful.
Why is it Important?
The Solvay process was a big step forward in chemistry. Before it, sodium carbonate was made using an older, more polluting method. Ernest Solvay's invention made the production of these chemicals much cleaner and more affordable.
Uses of Sodium Carbonate
Sodium carbonate, or soda ash, is used in many everyday products.
- Glassmaking: It helps lower the melting point of sand, making it easier to create glass for windows, bottles, and jars.
- Detergents and Soaps: It helps clean clothes and dishes by softening water and removing grease.
- Paper Manufacturing: It's used in making paper products.
- Water Treatment: It helps treat water for various uses.
Uses of Sodium Bicarbonate
Sodium bicarbonate, or baking soda, also has many uses.
- Baking: It's a common ingredient in baking, helping cakes and breads rise.
- Cleaning: It's used as a gentle abrasive cleaner and deodorizer.
- Fire Extinguishers: It can be found in some fire extinguishers.
History of the Solvay Process
Before the Solvay process, sodium carbonate was made using the Leblanc process. This older method produced a lot of pollution, including harmful gases and waste products.
Ernest Solvay's Innovation
Ernest Solvay was a Belgian industrial chemist. He developed his new process in the 1860s. He built his first factory in Couillet, Belgium, in 1863. His method was much more efficient and environmentally friendly than the Leblanc process. It quickly became the main way to produce sodium carbonate worldwide.
Impact on Industry
The Solvay process made sodium carbonate widely available and inexpensive. This helped many industries grow, especially those making glass, soap, and detergents. It also led to the growth of new towns and factories around the world, like the one in Solvay, New York.
Related pages
Images for kids
See also
 In Spanish: Proceso Solvay para niños
In Spanish: Proceso Solvay para niños
 | DeHart Hubbard |
 | Wilma Rudolph |
 | Jesse Owens |
 | Jackie Joyner-Kersee |
 | Major Taylor |