Iodine–starch test facts for kids
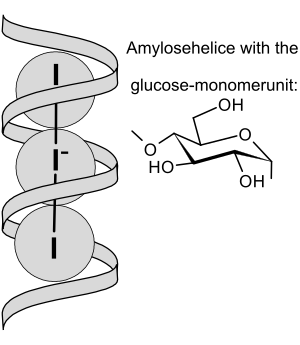
Schematic view of I3− ions embedded in amylose helix
|
|
| Classification | Colorimetric method |
|---|---|
| Analytes | Starch |
The iodine–starch test is a cool chemical trick. It helps us find out if starch or iodine is present in something. When starch and iodine mix, they create a super dark blue-black color.
This special reaction, where starch meets a tiny iodine particle called the triiodide anion (I−
3), is super important in chemistry. It's used in a method called iodometry, which helps scientists measure how much of certain chemicals are in a sample.
How the Iodine-Starch Test Works
Scientists J. J. Colin, H. F. Gaultier de Claubry, and F. Stromeyer first described this test in 1814.
When the triiodide anion touches starch, it instantly makes a strong blue-black color. This color gets lighter if the temperature goes up. It also gets lighter if you add liquids like ethanol that mix with water.
You can't do this test in very acidic conditions. This is because the starch would break down, which chemists call hydrolysis. Scientists believe that the iodine and iodide mixture joins with the starch. They form a long chain-like structure that creates the deep color.
Starch as a Chemical Detector
Starch is often used in chemistry as a special "indicator." It helps show when a chemical reaction, called a redox titration, is finished. This is especially true when triiodide is involved.
Starch forms a very dark blue-black compound with triiodide. But it only forms this color if both iodine and iodide (I−) are present. If only one of them is there, you won't see the color change.
The color of the starch complex is so strong that you can see it even if there's only a tiny bit of iodine. For example, you can see it when the iodine concentration is as low as 20 µM (a very small amount) at 20 °C.
When chemists are doing iodine titrations, they usually add the starch indicator near the end. If they add it too early, the dark starch–triiodide complex might form too much. This could stop some of the iodine from reacting properly. So, they wait until most of the iodine has reacted before adding the starch.
This color change can also be used to find moisture or sweat. One example is in the Minor test, which is also known as the starch–iodine test.
See also
 In Spanish: Prueba del yodo para niños
In Spanish: Prueba del yodo para niños
- Lugol's iodine
- Counterfeit banknote detection pen
 | DeHart Hubbard |
 | Wilma Rudolph |
 | Jesse Owens |
 | Jackie Joyner-Kersee |
 | Major Taylor |



