Coordination complex facts for kids
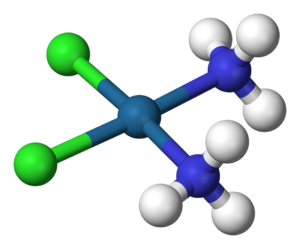
A coordination complex is a special type of chemical compound. It has a central atom, usually a metal, that is connected to other molecules or ions. These attached parts are called ligands. Think of the central metal atom as the "boss" and the ligands as its "helpers" or "friends." They all work together to form a unique structure.
Contents
What are Coordination Complexes?
Imagine a tiny metal atom, like iron or copper, at the very center. This is the central atom. Around it, other molecules or ions gather. These are the ligands. Ligands have extra electrons they can share. They use these electrons to "hold hands" with the central metal atom. This "hand-holding" is a special chemical bond. It creates the coordination complex.
The Central Atom and Ligands
The central atom is often a transition metal. These metals are good at forming many bonds. They have empty spaces in their electron clouds. This allows them to accept electrons from the ligands.
Ligands can be many different things. They might be simple water molecules. They could also be ammonia (NH3) or chloride ions (Cl-). Each ligand has at least one pair of electrons. These are called "lone pairs." They are ready to be shared with the central metal atom.
How They Form Bonds
The bond between the central atom and the ligand is called a "coordinate bond." It's a bit like a regular chemical bond. But in this case, both shared electrons come from the ligand. The ligand "donates" its electron pair to the central metal atom. This makes the complex stable.
Why are Coordination Complexes Important?
Coordination complexes are everywhere! They are super important in nature and in our daily lives. They play many different roles.
In Nature
Many living things rely on these complexes. For example, hemoglobin in your blood is a coordination complex. It has an iron atom at its center. This iron atom binds to oxygen. This is how oxygen travels through your body. Without it, we couldn't breathe!
Another example is chlorophyll. This is the green stuff in plants. Chlorophyll has a magnesium atom at its core. It helps plants capture sunlight. This energy is then used to make food.
In Medicine and Industry
Coordination complexes are also very useful for people. Doctors use them as medicines. For instance, a complex called cisplatin helps treat some types of cancer. It works by stopping cancer cells from growing.
In factories, these complexes act as catalysts. Catalysts speed up chemical reactions. They help make plastics, fuels, and many other products. They are also used to clean up pollution. Some complexes can grab harmful metals from water.
Discovering Coordination Complexes
The idea of coordination complexes is not new. A scientist named Alfred Werner was key to understanding them. He did his work over 100 years ago.
Alfred Werner's Work
Alfred Werner was a Swiss chemist. He studied many different compounds. He noticed that some metal salts behaved strangely. They seemed to have more atoms attached than expected.
Werner proposed that metal atoms could bond with a specific number of other molecules. He called these "coordination numbers." His ideas helped explain how these complexes were structured. He even predicted new types of complexes. His work was so important that he won a Nobel Prize in Chemistry in 1913.
Images for kids
-
Alfred Werner, the scientist who helped us understand coordination complexes.
 | Dorothy Vaughan |
 | Charles Henry Turner |
 | Hildrus Poindexter |
 | Henry Cecil McBay |