Alfred Werner facts for kids
Quick facts for kids
Alfred Werner
|
|
|---|---|
 |
|
| Born | 12 December 1866 |
| Died | 15 November 1919 (aged 52) |
| Nationality | Swiss |
| Alma mater | University of Zurich ETH Zurich |
| Known for | configuration of transition metal complexes |
| Awards | Nobel Prize for Chemistry (1913) |
| Scientific career | |
| Fields | Inorganic chemistry |
| Institutions | University of Zurich |
| Doctoral advisor | Arthur Rudolf Hantzsch, Marcellin Berthelot |
Alfred Werner was a brilliant Swiss chemist who made huge discoveries about how atoms connect to form special chemical compounds. He won the Nobel Prize in Chemistry in 1913 for his ideas about the shapes of these compounds, especially those involving transition metals. His work laid the groundwork for a whole new area of chemistry called coordination chemistry. He was the first inorganic chemist to win the Nobel Prize.
Contents
Biography
Alfred Werner was born on December 12, 1866, in Mulhouse, a city that was part of France at the time. He was the youngest of four children. He later moved to Switzerland to study chemistry at the Swiss Federal Institute in Zurich.
Since this institute could not give out doctorates until 1909, Werner officially received his doctorate from the University of Zurich in 1890. After studying further in Paris, he returned to the Swiss Federal Institute to teach in 1892. In 1893, he moved to the University of Zurich, where he became a professor in 1895. He became a Swiss citizen in 1894.
Alfred Werner passed away in Zurich on November 15, 1919, after a period of illness.
Werner's Chemical Discoveries
Understanding Coordination Chemistry
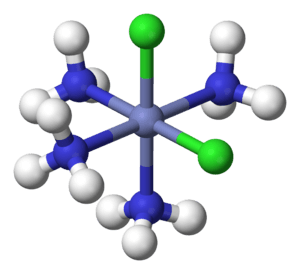
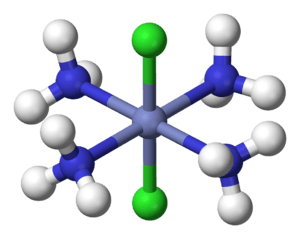
In 1893, Werner was the first to correctly describe the structures of special chemical compounds called coordination compounds. These compounds have a central metal atom, often a transition metal, surrounded by other atoms or groups of atoms called ligands.
For example, chemists knew about a compound called hexamminecobalt(III) chloride, which looked like CoCl3•6NH3. The dot in the formula made its structure a mystery. Werner suggested that the Co3+ ion was at the center, surrounded by six NH3 (ammonia) molecules. These six ammonia molecules were arranged like the points of an octahedron. The three Cl− (chloride) ions were separate, like free ions in a solution.
Werner proved his idea by measuring how well the compound conducted electricity in water. He also showed that the chloride ions could be easily removed. Later, other tests also confirmed his ideas.
Explaining Isomers
Werner also helped explain why some compounds with the same chemical formula could exist in different forms, called isomers. These isomers have the same atoms but are arranged differently in space.
For example, there were two forms of "Co(NH3)4Cl3" – one green and one purple. Werner showed that these were actually two different geometric isomers of the compound [Co(NH3)4Cl2]Cl. In both, the cobalt atom is surrounded by four NH3 and two Cl ligands, forming an octahedron.
- The green isomer is "trans," meaning the two Cl ligands are on opposite sides of the cobalt atom.
- The purple isomer is "cis," meaning the two Cl ligands are next to each other.
Werner also made compounds that were optical isomers. These are like your left and right hands – they are mirror images of each other but cannot be perfectly placed on top of each other. In 1914, he created the first such compound that did not contain carbon, called hexol.
Understanding Chemical Bonds
Before Werner, chemists described the "valence" of an element as simply the number of bonds it could form. Werner introduced a new way of thinking about bonds in coordination compounds.
He suggested that there were two types of "valence":
- Primary valence: This was like the main charge of the metal ion, which could be balanced by other ions at a distance. In modern terms, this is called the oxidation state.
- Secondary valence: This described the number of atoms or molecules directly attached to the central metal atom. He called this the coordination number. For example, in [Co(NH3)6]Cl3, the coordination number of cobalt is 6 because six ammonia molecules are directly attached to it.
Werner's ideas helped other scientists, like Richard Abegg and Gilbert N. Lewis, develop important rules about how atoms bond, such as the "octet rule" which explains why atoms often try to have eight electrons in their outer shell.
Today, we understand that Werner's primary valence refers to ionic bonds, and his secondary valence describes coordinate covalent bonds, where one atom donates both electrons to form a bond with another.
See also
 In Spanish: Alfred Werner para niños
In Spanish: Alfred Werner para niños
 | John T. Biggers |
 | Thomas Blackshear |
 | Mark Bradford |
 | Beverly Buchanan |