Gilbert N. Lewis facts for kids
Quick facts for kids
Gilbert N. Lewis
|
|
|---|---|
 |
|
| Born | October 23, 1875 or October 25, 1875 |
| Died | March 23, 1946 (aged 70) |
| Nationality | American |
| Known for | Lewis pair Lewis structures Lewis acids and bases Lewis–Tolman paradox Chemical thermodynamics Valence bond theory Covalent bond Cubical atom Fugacity Heavy water Ionic strength Octet rule Tetraoxygen Thermodynamic activity Named photon Explained phosphorescence |
| Awards | Fellow of the Royal Society William H. Nichols Medal (1921) Willard Gibbs Award (1924) Davy Medal (1929) |
| Scientific career | |
| Fields | Physical chemist |
| Thesis | A general equation for free energy and physico-chemical equilibrium, and its application (1899) |
| Doctoral advisor | Theodore William Richards |
| Doctoral students | Michael Kasha Harold Urey Glenn T. Seaborg Joseph Edward Mayer |
| Influences | Irving Langmuir Merle Randall |
Gilbert Newton Lewis (October 23 or 25, 1875 – March 23, 1946) was an American physical chemist. He was also a dean at the College of Chemistry at the University of California, Berkeley. Lewis is famous for discovering the covalent bond, which is when atoms share electrons. His ideas, like Lewis dot structures, greatly changed how we understand chemical bonding today.
Lewis also made important contributions to chemical thermodynamics (how energy changes in chemical reactions), photochemistry (how light affects chemical reactions), and isotope separation (separating different forms of atoms). He also developed a new way to define acids and bases. In 1926, he even came up with the word "photon" for the tiny particles of light.
Gilbert N. Lewis was born in 1875 in Weymouth, Massachusetts. After getting his PhD in chemistry from Harvard University and studying in Germany and the Philippines, Lewis moved to California in 1912. He taught chemistry at the University of California, Berkeley, where he became the Dean of the College of Chemistry. He spent the rest of his life there. As a professor, he made chemical thermodynamics easier for other chemists to understand. He also started measuring the free energy for many chemical processes. In 1916, he shared his ideas about how atoms bond together. Later, in 1933, he began researching isotope separation. He worked with hydrogen and was the first to purify a sample of heavy water. Lewis also developed his theory of acids and bases and studied photochemistry in his later years.
Even though he was nominated 41 times, G. N. Lewis never won the Nobel Prize in Chemistry. This led to some debate among scientists. However, Lewis guided and inspired many future Nobel Prize winners at Berkeley. These included Harold Urey (who won in 1934), William F. Giauque (1949), Glenn T. Seaborg (1951), Willard Libby (1960), and Melvin Calvin (1961). Because of his work, Berkeley became a world-leading center for chemistry. Lewis died on March 23, 1946, in his Berkeley lab. Today, Lewis Hall on the Berkeley campus is named in his honor.
Contents
Biography
Early life and education
Gilbert N. Lewis was born in 1875 in Weymouth, Massachusetts. He was a very smart child and learned to read at age three. He was taught at home by his parents. In 1884, his family moved to Lincoln, Nebraska. He got his first formal schooling there in 1889 at a university prep school.
In 1893, Lewis went to the University of Nebraska for two years. Then he transferred to Harvard University, where he earned his bachelor's degree in 1896. After teaching for a year, Lewis returned to Harvard to study with chemist T. W. Richards. He earned his PhD in 1899. After another year of teaching at Harvard, Lewis traveled to Germany. Germany was a major center for physical chemistry at the time. He studied with famous chemists Walther Nernst and Wilhelm Ostwald.
Career path
After his studies in Germany, Lewis returned to Harvard in 1901 as an instructor. In 1904, he took a break to work in the Philippines as the Superintendent of Weights and Measures. The next year, he came back to the United States. He joined the Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts. There, he worked with other great physical chemists. He became a full professor at MIT in 1911.
University of California, Berkeley
In 1912, G. N. Lewis moved to the University of California, Berkeley. He became a professor of physical chemistry and the Dean of the College of Chemistry. In 1912, he married Mary Hinckley Sheldon. They had two sons and a daughter. Both of their sons later became chemistry professors.
At Berkeley, Lewis was a mentor to many students who later won the Nobel Prize. These included Harold Urey, William F. Giauque, Glenn T. Seaborg, Willard Libby, and Melvin Calvin. Thanks to his leadership, the College of Chemistry at Berkeley became one of the best chemistry centers in the world. Lewis was elected to the National Academy of Sciences in 1913. He later resigned in 1934. Some people believe he resigned because his student, Harold Urey, won the Nobel Prize for discovering deuterium, a prize Lewis felt he should have shared.
Death
On March 23, 1946, Gilbert Lewis was found dead in his laboratory at Berkeley. He had been working with liquid hydrogen cyanide. Deadly fumes from a broken line had leaked into the lab. The official cause of death was listed as coronary artery disease.
Lewis is buried in Golden Gate National Cemetery. Lewis Hall at Berkeley, built in 1948, is named in his honor.
Scientific achievements
Thermodynamics
Lewis became very interested in thermodynamics during his time at Harvard. Thermodynamics is the study of how heat and energy relate to other forms of energy and work. By 1895, many important thermodynamic equations were known, but they weren't organized into a clear system. Also, these equations only worked for ideal chemical systems, not real ones. Lewis wanted to fix these problems.
In 1900 and 1901, Lewis introduced the ideas of "activity" and "fugacity" in thermodynamics. He described fugacity as an "escaping tendency." This meant it showed how likely a substance was to move from one chemical state to another. Lewis hoped this idea would help create a better system for understanding real chemical reactions. While his full system wasn't adopted, the idea of fugacity is still used today to describe real gases.
Lewis also understood the importance of free energy and entropy in chemistry. These concepts help predict if a chemical change will happen. He spent much of his career making these complex ideas easier for other chemists to use.
Valence theory
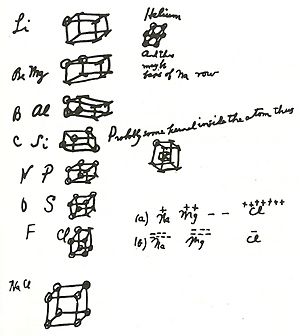
Around 1902, Lewis started drawing "cubical atoms" in his lecture notes. In these drawings, the corners of the cube showed where electrons might be. He later said these notes were the first time he shared his ideas about how atoms bond.
Lewis's "cubic atom" idea helped explain why the periodic table has cycles of eight elements. It also fit with the idea that chemical bonds form when atoms share or transfer electrons to get a full set of eight. This idea was an important part of his 1916 paper on chemical bonding.
In 1916, Lewis published his famous paper, "The Atom and the Molecule." In it, he introduced the idea of the covalent bond. This is a bond where atoms share a pair of electrons. He also defined "odd molecules" (now called free radicals), which are molecules with an unpaired electron. His paper included what are now known as Lewis dot structures, along with his cubical atom model. These ideas were later expanded by other scientists and greatly influenced our understanding of chemical bonds.
Acids and bases
In 1923, Lewis developed his electron-pair theory of acid–base reactions. In this theory, a "Lewis acid" is a substance that can accept an electron pair. A "Lewis base" is a substance that can donate an electron pair. This was a new way to define acids and bases, different from earlier definitions.
Lewis spent 25 years studying the free energy of different substances. In 1923, he and Merle Randall published their findings. This work helped create modern chemical thermodynamics.
Heavy water
Lewis was the first to create a pure sample of deuterium oxide, also known as heavy water, in 1933. He was also the first to study how living things survived and grew in heavy water. By speeding up deuterons (nuclei of deuterium atoms) in a cyclotron, he was able to learn more about atomic nuclei. In the 1930s, he mentored Glenn T. Seaborg, who later won the 1951 Nobel Prize in Chemistry.
Tetraoxygen (O4)
In 1924, Lewis studied the magnetic properties of oxygen dissolved in liquid nitrogen. He discovered that O4 molecules were forming. This was the first time anyone found evidence of tetratomic oxygen, which is a molecule made of four oxygen atoms.
Relativity and quantum physics

In 1908, Lewis published a paper on relativity. In it, he found a different way to explain the relationship between mass and energy than Albert Einstein had. In 1909, he and Richard C. Tolman combined his methods with special relativity.
In 1926, Lewis coined the term "photon" for the smallest unit of radiant energy, or light. He wrote a letter to the journal Nature suggesting this name. While his theory about photons was different from Einstein's, his name for the "light quantum" stuck.
Other achievements
In 1921, Lewis was the first to propose an equation that described why strong electrolytes didn't always follow the law of mass action. This was a problem that had puzzled chemists for many years. His equations for what he called ionic strength were later confirmed by other scientists.
Lewis also published on many other topics, from the nature of light to economics. In his later years, Lewis and his student Michael Kasha discovered that phosphorescence (when a substance glows after being exposed to light) in organic molecules involves light coming from an electron in a special "triplet state."
See also
 In Spanish: Gilbert N. Lewis para niños
In Spanish: Gilbert N. Lewis para niños
Images for kids