Lewis structure facts for kids
Lewis structures are like simple maps that show how atoms connect and share their tiny electrons to form molecules. They are also called Lewis-dot diagrams or electron dot diagrams. These diagrams help us understand how atoms stick together and what shape molecules might have.
In a Lewis structure, each dot stands for one electron. When you see two dots side by side, it means there's a "lone pair" of electrons. These are electrons that belong to an atom but aren't being shared with another atom to form a bond. A line between two atoms shows a pair of shared electrons. This shared pair is what creates a chemical bond, holding the atoms together.
Contents
What are Lewis Structures?
Lewis structures are visual tools used in chemistry. They help scientists and students see how atoms are arranged in a molecule. They also show how electrons are distributed around these atoms. This is important because the way electrons are shared or not shared affects how a molecule behaves.
These diagrams were first introduced by an American chemist named Gilbert N. Lewis in 1916. He came up with this simple way to represent the valence electrons of an atom. Valence electrons are the electrons in the outermost shell of an atom. These are the electrons that are involved in forming chemical bonds.
Why are Lewis Structures Important?
Understanding Lewis structures helps us predict how molecules will react with each other. They show us which atoms are connected and if there are any unshared electrons. These unshared electrons, called lone pairs, can play a big role in how a molecule interacts with other molecules.
For example, the shape of a molecule can affect its properties. Lewis structures give us clues about a molecule's shape. This knowledge is used in many fields, from designing new medicines to understanding how our bodies work.
How to Draw Simple Lewis Structures
Drawing a Lewis structure involves a few simple steps. The main idea is to make sure each atom in the molecule has a stable number of electrons around it. For most atoms, this means having eight valence electrons, which is called the "octet rule." Hydrogen is an exception, needing only two electrons.
Here are the basic steps:
- Step 1: Count Total Valence Electrons. First, find out how many valence electrons each atom in the molecule has. You can usually find this from the atom's position on the periodic table. Then, add them all up to get the total number of electrons you'll use in your drawing.
- Step 2: Place the Central Atom. Usually, the least electronegative atom (the one that doesn't pull electrons as strongly) goes in the middle. Hydrogen atoms are almost never central atoms.
- Step 3: Draw Single Bonds. Connect the central atom to the other atoms with single lines. Each line represents two shared electrons. Subtract these electrons from your total count.
- Step 4: Add Lone Pairs. Place the remaining electrons as lone pairs on the outer atoms first, making sure each outer atom (except hydrogen) gets eight electrons.
- Step 5: Place Remaining Electrons on Central Atom. If you still have electrons left, put them on the central atom as lone pairs.
- Step 6: Check for Octets. If the central atom doesn't have eight electrons, you might need to form double or triple bonds. Do this by moving a lone pair from an outer atom to become a shared pair between the central and outer atom.
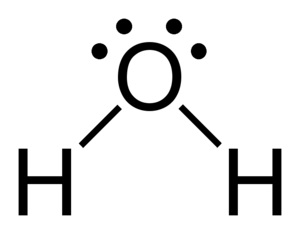
Example: Water (H2O)
Let's look at water, H₂O.
- Oxygen (O) has 6 valence electrons.
- Each Hydrogen (H) has 1 valence electron.
- Total valence electrons = 6 (from O) + 1 (from H) + 1 (from H) = 8 electrons.
Now, let's draw it:
- Oxygen is the central atom.
- Connect the two Hydrogen atoms to the Oxygen with single bonds. That uses 4 electrons (2 bonds x 2 electrons/bond).
- You have 8 - 4 = 4 electrons left.
- Place these 4 electrons as two lone pairs on the Oxygen atom.
- Now, Oxygen has 2 shared pairs (4 electrons) + 2 lone pairs (4 electrons) = 8 electrons. Each Hydrogen has 1 shared pair (2 electrons). Everyone is happy!
Images for kids
-
A skeletal diagram of butane
See also
 In Spanish: Estructura de Lewis para niños
In Spanish: Estructura de Lewis para niños
 | Percy Lavon Julian |
 | Katherine Johnson |
 | George Washington Carver |
 | Annie Easley |