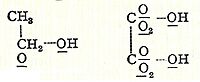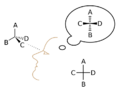History of molecular theory facts for kids
Have you ever wondered how tiny particles called atoms stick together to form everything around us? The history of molecular theory is all about how people slowly figured out that atoms can join up to make larger, stable groups called molecules.
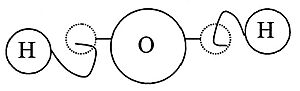
For a long time, people didn't know about molecules. But over hundreds of years, scientists did experiments and came up with ideas. They learned that different atoms, like hydrogen and oxygen, can combine in special ways to create molecules, such as water.
Contents
Early Ideas
The idea of tiny building blocks goes way back! Ancient Greek thinkers like Leucippus and Democritus believed that everything in the universe was made of super small, uncuttable pieces called atoms, with empty space in between them.
Around 450 BC, a philosopher named Empedocles thought there were four basic elements: fire (![]() ), earth (
), earth (![]() ), air (
), air (![]() ), and water (
), and water (![]() ). He also believed in "forces" that pulled these elements together or pushed them apart.
). He also believed in "forces" that pulled these elements together or pushed them apart.
Later, Plato thought that mathematical shapes were the real building blocks of the world. He saw the four elements as different states of these shapes. A fifth element, called aether, was thought to make up the stars and planets. These ideas were accepted by Aristotle and influenced thinking for many centuries.
How Greek Thinkers Imagined Atoms
The earliest ideas about how atoms might connect came from Leucippus, Democritus, and Epicurus. They thought that how solid a material was depended on the shape of its atoms. For example:
- Iron atoms were solid and strong, with hooks that made them lock together.
- Water atoms were smooth and slippery.
- Salt atoms were sharp and pointed, which explained their taste.
- Air atoms were light and whirring, filling all other materials.
Democritus was a big supporter of these ideas. He used everyday examples to describe atoms. He said atoms were different based on their shape, size, and how their parts were arranged. He even imagined that atoms had special "attachments" like hooks and eyes, or balls and sockets, to connect with each other.
New Discoveries in the 1600s
After the Roman Empire declined, the idea of atoms was mostly forgotten for a long time. People focused more on theories about the four elements or alchemy. But in the 1600s, the atomic theory made a comeback, thanks to thinkers like Pierre Gassendi and Isaac Newton.
Gassendi studied ancient ideas and brought them back. He thought that the size and shape of atoms moving in empty space could explain why matter had certain properties. For instance, he believed heat came from small, round atoms, and cold from sharp, pointed atoms. He also thought solids were held together by atoms with hooks that intertwined.
Newton, a very famous scientist, also believed that particles attracted each other with a strong force when they were very close. He wrote in his book Opticks that this force caused chemical reactions.
A big step towards understanding molecules came from Robert Boyle in 1661. In his book The Sceptical Chymist, he suggested that matter was made of "clusters of particles." He thought that chemical changes happened when these clusters rearranged themselves. Boyle called these basic particles "corpuscles" and said they came in different types and sizes.
In 1680, a French chemist named Nicolas Lemery used Boyle's ideas. He said that acidic substances had pointed particles, while alkaline substances had pores (small holes). According to him, a molecule was formed when these pointed particles fit into the pores, like a lock and key.
The 1700s: Forces and Connections
In the 1700s, scientists started thinking about "chemical affinity." This was an early idea about how atoms might combine. For example, in 1718, French chemist Étienne François Geoffroy built on Boyle's ideas. He created "affinity tables" that showed how different substances were attracted to each other. These tables were popular for many years.
In 1738, Daniel Bernoulli, a Swiss scientist, published a book called Hydrodynamica. In it, he suggested that gases are made of many molecules moving in all directions. He said that when these molecules hit a surface, they create the gas pressure we feel. He also proposed that what we call heat is simply the energy of these molecules moving around. This was an early idea of the kinetic theory of gases.
Later, in 1789, William Higgins wrote about how "ultimate" particles (his term for atoms) combined. He even hinted at the idea of valency bonds, which describe how many connections an atom can make. He thought that if the force between oxygen and nitrogen was 6, it would be divided among their connections.
The 1800s: Molecules Take Shape
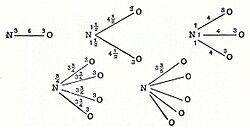
In 1803, John Dalton developed his own atomic theory. He used hydrogen as a starting point and figured out the ratios in which other elements combined. For example, he found that nitrous anhydride had a 2 to 3 ratio of nitrogen to oxygen, giving it the formula N2O3. Dalton also imagined atoms "hooking" together to form molecules. In 1808, he published famous diagrams of these combined "atoms."
The word "molecule" itself was created by Amedeo Avogadro. In his 1811 paper, he explained that the smallest particles of gases are not always single atoms. Instead, they can be made of several atoms joined together by attraction to form a single "molecule." Avogadro used the term "elementary molecule" for atoms and "compound molecules" for groups of atoms.
While in Italy, Avogadro came up with what we now call Avogadro's law. This law states that if you have equal volumes of different gases at the same temperature and pressure, they will contain the same number of molecules. This was a huge step because it meant scientists could now figure out the relative weights of molecules from gas samples.
Avogadro's idea helped solve a big problem: the confusion between atoms and molecules. He clearly showed that even simple particles could be made of molecules, and these molecules were made of atoms. Dalton, on the other hand, didn't think this was possible.
In 1826, French chemist Jean-Baptiste Dumas supported Avogadro's ideas. He stated that gases under similar conditions are made of molecules or atoms placed at the same distance from each other.
In 1833, another French chemist, Marc Antoine Auguste Gaudin, made Avogadro's idea even clearer. He used "volume diagrams" that showed how molecules looked in the gas phase. These diagrams even showed some correct molecular shapes, like water (H2O), though he drew it as a straight line.
In the mid-1800s, scientists started to understand how atoms connect within molecules. In 1857-58, Friedrich August Kekulé was the first to suggest how every atom in an organic molecule was bonded to others. He proposed that carbon atoms could form four bonds and link up with themselves to create the "skeletons" of organic molecules.
In 1856, Scottish chemist Archibald Couper also developed a similar theory of molecular structure. He had a very clear idea of how molecules were built, suggesting that atoms joined together like parts of a toy building set, forming specific 3D shapes. Couper was the first to use lines between atoms to show bonds, which is how we draw them today. He also suggested that some molecules formed straight chains, while others, like tartaric acid, formed rings.
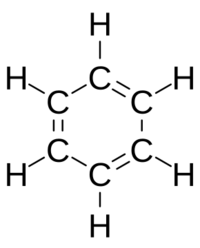
In 1861, a high-school teacher named Joseph Loschmidt published a booklet with amazing early pictures of molecules. He showed "ringed" structures and even double bonds, like in ethylene (H2C=CH2) and acetylene (HC≡CH).
Loschmidt also suggested a possible shape for benzene, but it was Kekulé who proposed the modern ring structure for benzene in 1865. The ring shape of benzene was later confirmed by scientist Kathleen Lonsdale. Benzene is special because its carbon atoms have alternating double bonds to make the ring stable.
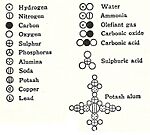
In 1865, German chemist August Wilhelm von Hofmann was the first to make "stick-and-ball" models of molecules. He used them in his lectures to show how atoms connect. For example, he showed a model of methane (CH4).

Hofmann's models followed an idea from his colleague William Odling that carbon atoms can form four bonds. Hofmann's color scheme for atoms is still used today: carbon is black, nitrogen is blue, oxygen is red, chlorine is green, sulfur is yellow, and hydrogen is white. However, his models weren't perfect. They showed carbon bonds as flat, not 3D, and the atoms were not to scale.
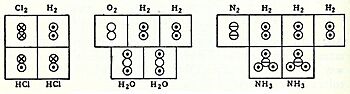
In 1864, Scottish chemist Alexander Crum Brown started drawing molecules by putting atom symbols in circles and using broken lines to connect them.
The year 1873 was very important for the idea of the "molecule." The famous Scottish physicist James Clerk Maxwell wrote an article called 'Molecules' in Nature magazine. In it, Maxwell clearly stated: "An atom is a body which cannot be cut in two; a molecule is the smallest possible portion of a particular substance."
Maxwell explained that the word "molecule" was new and that the ideas behind it belonged to modern chemistry. He said that atoms were like points with "potential forces" around them. He also explained that when "flying molecules" hit a solid object, it creates the pressure we feel from air and other gases. However, he noted that no one had ever actually seen a molecule.
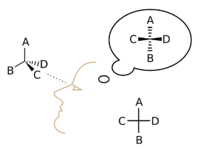
In 1874, Jacobus Henricus van 't Hoff and Joseph Achille Le Bel independently suggested that a molecule's ability to rotate light (called optical activity) could be explained if carbon atoms and their neighbors were connected towards the corners of a 3D shape called a tetrahedron. This helped scientists understand the 3D nature of molecules much better.
Emil Fischer later developed a way to draw 3D molecules on a flat piece of paper, called the Fischer projection.
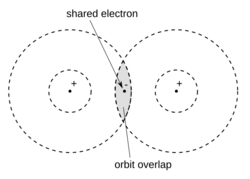
In 1898, Ludwig Boltzmann, in his Lectures on Gas Theory, used the idea of valence (how many bonds an atom can form) to explain how gas molecules can break apart into atoms at high temperatures. He drew some of the first simple pictures of how atomic orbitals (the areas where electrons are found) overlap. Boltzmann suggested that atoms have "sensitive regions" on their surface. When these regions touch or overlap, a chemical attraction forms, and the atoms become "chemically bound."
The 1900s: Electrons and Bonds
In the early 1900s, American chemist Gilbert N. Lewis started using dots in his lectures at Harvard to represent the electrons around atoms. His students liked these drawings. Lewis noticed that elements with a certain number of electrons seemed to be very stable. This idea was called "Abegg's law of valence" (now known as Abegg's rule). Lewis thought that once an atom had eight electrons in its outer shell, that shell was full, and a new one would start. He also noticed that many ions (charged atoms) with eight electrons were very stable. This led him to propose the octet rule: Atoms or ions with a full outer layer of eight electrons are especially stable.
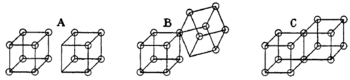
Lewis imagined an atom as a cube with eight corners, each available for an electron. He thought that atoms could bond by sharing edges of these cubes to form "cubic-structured molecules."
In this model, when two atoms shared an edge, they shared two electrons, forming an electron-pair bond (like structure C above). If an electron moved from one cube to another, it formed a charged ionic bond (like A). Double bonds were formed by sharing a whole face between two cubic atoms, meaning four electrons were shared.
In 1913, Lewis read a paper by a student named Alfred Lauck Parson. Parson suggested that electrons were not just charges but also tiny magnets, and that a chemical bond happened when two electrons were shared between two atoms. Lewis realized this meant bonding occurred when two electrons formed a shared edge between two complete cubes.
Based on these ideas, Lewis published his famous 1916 article, The Atom and the Molecule. In it, he introduced "Lewis structures" to show atoms and molecules. In these drawings, dots represent electrons, and lines represent covalent bonds. He developed the idea of the electron-pair bond, where two atoms can share one to six electrons, forming single bonds, double bonds, or triple bonds.
Lewis explained: "An electron may form a part of the shell of two different atoms and cannot be said to belong to either one exclusively." He also said that atoms tried to gain or lose electrons to complete their "cube" of eight electrons. Lewis structures show each atom with its chemical symbol. Lines connect bonded atoms, or sometimes pairs of dots are used. Extra electrons that don't form bonds are shown as pairs of dots next to the atom.
Lewis summed up his new bonding model: "Two atoms may follow the rule of eight, or the octet rule, not only by moving electrons from one atom to another, but also by sharing one or more pairs of electrons...Two electrons linked together in this way, when they are between two atomic centers and held jointly in the shells of both atoms, I have considered to be the chemical bond."
In 1917, a young student named Linus Pauling was learning about the old "hook-and-eye" bonding method. He wasn't happy with this old idea and started looking into the new field of quantum physics for a better way to understand bonds.
In 1927, physicists Fritz London and Walter Heitler used the new quantum mechanics to explain the forces between atoms in a hydrogen molecule. Their work was a major breakthrough and influenced Pauling, who later visited them.
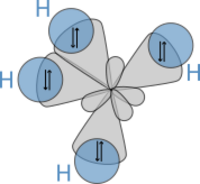
In 1931, building on their work and Lewis's ideas, Pauling published his important article "The Nature of the Chemical Bond." In it, he used quantum mechanics to figure out the properties and structures of molecules, like the angles between bonds. Pauling developed hybridization theory to explain bonds in molecules like methane (CH4). In methane, four special "hybrid" orbitals from carbon overlap with hydrogen's orbitals, creating four strong bonds of the same length and strength. This gives methane its specific 3D shape.
For his amazing theories, Pauling won the Nobel Prize in Chemistry in 1954. He is the only person to win two unshared Nobel Prizes, also receiving the Nobel Peace Prize in 1963.
In 1926, French physicist Jean Perrin won the Nobel Prize in physics for proving that molecules really exist. He did this by calculating Avogadro number (the number of molecules in a certain amount of substance) using three different methods, all involving liquids.
In 1937, chemist K.L. Wolf introduced the idea of "supermolecules" to describe how molecules like acetic acid can form larger groups through hydrogen bonding. This led to the field of supermolecular chemistry, which studies how molecules interact without forming strong chemical bonds.
In 1951, physicist Erwin Wilhelm Müller invented the field ion microscope. This allowed him to be the first person to actually see individual atoms and how they were arranged in a metal tip.
In 2009, scientists from IBM made history by taking the first picture of a real molecule! Using an atomic force microscope, they were able to image every single atom and bond of a pentacene molecule.
Images for kids
See also
 In Spanish: Historia de la teoría molecular para niños
In Spanish: Historia de la teoría molecular para niños
- History of chemistry
- Atomic theory
- Kinetic theory of gases
 | William Lucy |
 | Charles Hayes |
 | Cleveland Robinson |