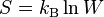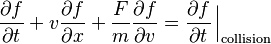Ludwig Boltzmann facts for kids
Quick facts for kids
Ludwig Boltzmann
|
|
|---|---|
 |
|
| Born |
Ludwig Eduard Boltzmann
20 February 1844 |
| Died | 5 September 1906 (aged 62) Tybein, Triest, Austria-Hungary
|
| Alma mater | University of Vienna |
| Known for |
|
| Awards | ForMemRS (1899) |
| Scientific career | |
| Fields | Physics |
| Institutions |
|
| Doctoral advisor | Josef Stefan |
| Other academic advisors |
|
| Doctoral students |
|
| Other notable students |
|
| Signature | |
Ludwig Eduard Boltzmann (born February 20, 1844 – died September 5, 1906) was an Austrian physicist and philosopher. He is best known for developing statistical mechanics. This field helps explain how the tiny parts of matter behave. He also helped explain the second law of thermodynamics using statistics.
In 1877, Boltzmann gave us the modern definition of entropy. Entropy is a measure of how much disorder there is in a system. He used the formula: 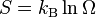 . Here, Ω shows how many ways a system's energy can be arranged. The constant kB is now called the Boltzmann constant.
. Here, Ω shows how many ways a system's energy can be arranged. The constant kB is now called the Boltzmann constant.
Statistical mechanics is a key part of modern physics. It links what we see on a large scale (like temperature and pressure) to what happens at a tiny, microscopic level. It helps us understand how things like heat capacity relate to the behavior of atoms and molecules. Before this, scientists had to measure these things for every material.
Contents
Biography
Early Life and Education
Ludwig Boltzmann was born in Erdberg, a town near Vienna. His father, Ludwig Georg Boltzmann, worked for the government. His mother, Katharina Pauernfeind, was from Salzburg. Ludwig was taught at home when he was young.
He went to high school in Linz, Upper Austria. When he was 15, his father passed away. In 1863, Boltzmann began studying mathematics and physics at the University of Vienna. He earned his doctorate degree in 1866. In 1869, he became qualified to teach at universities. He worked closely with Josef Stefan, who was in charge of the physics institute. Stefan was the one who introduced Boltzmann to the ideas of James Clerk Maxwell.
University Career
In 1869, at just 25 years old, Boltzmann became a professor at the University of Graz. He taught Mathematical Physics. He spent time in other cities like Heidelberg and Berlin. There, he worked with famous scientists such as Robert Bunsen and Gustav Kirchhoff.
In 1873, Boltzmann moved back to the University of Vienna. He taught mathematics there until 1876.
In 1872, he met Henriette von Aigentler in Graz. She wanted to study math and physics but was not allowed to attend lectures. Boltzmann helped her appeal this decision, and she won. Ludwig Boltzmann and Henriette married on July 17, 1876. They had three daughters and one son.
Boltzmann returned to Graz to teach Experimental Physics. He was very happy there for 14 years. During this time, he developed his important ideas about nature based on statistics. Some of his students in Graz included Svante Arrhenius and Walther Nernst.
In 1890, Boltzmann became a professor at the University of Munich in Germany. Later, in 1894, he took over from his former teacher, Josef Stefan, as Professor of Theoretical Physics at the University of Vienna.
Later Years and Passing
In his last years, Boltzmann worked hard to defend his scientific ideas. He had disagreements with some colleagues in Vienna, especially Ernst Mach. In 1900, Boltzmann went to the University of Leipzig at the invitation of Wilhelm Ostwald.
After Mach retired, Boltzmann came back to Vienna in 1902. In 1903, he helped start the Austrian Mathematical Society. Among his students were Paul Ehrenfest and Lise Meitner.
In Vienna, Boltzmann taught physics and also gave lectures on philosophy. His lectures on natural philosophy were very popular. People even stood in the hallways to listen. The Emperor himself invited Boltzmann to a reception because of his successful lectures.
In 1906, Boltzmann's health declined, and he had to leave his job. He is buried in the Viennese Zentralfriedhof. His tombstone shows his famous Boltzmann's entropy formula: 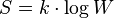 .
.
Boltzmann's Ideas on Physics
Boltzmann's most important work was in kinetic theory. This theory explains how gas particles move. He helped develop the Maxwell–Boltzmann distribution, which describes the speeds of molecules in a gas.
Maxwell–Boltzmann statistics and the Boltzmann distribution are still key parts of classical statistical mechanics today. They help us understand what temperature really means.
Many chemists, like John Dalton and James Clerk Maxwell, believed in atoms and molecules. However, many physicists did not believe they truly existed until much later. Boltzmann had long debates with the editor of a German physics journal. The editor would not let Boltzmann write about atoms and molecules as if they were real.
Just a few years after Boltzmann's death, Perrin's studies of tiny particles in liquids (called colloidal suspensions) proved that atoms and molecules really exist. This was based on Einstein's earlier work. These studies confirmed the values of the Avogadro constant and the Boltzmann constant.
Max Planck said that Boltzmann was the first to connect entropy and probability. This famous formula for entropy S is:
Here, kB is the Boltzmann constant. The "ln" means the natural logarithm. W stands for the number of ways a system can be arranged at a microscopic level. It's like the number of different ways you can arrange molecules in a gas.
Boltzmann also suggested in 1877 that the energy levels of a physical system could be separate, or "discrete." This idea was a step towards quantum mechanics.
The Boltzmann Equation
The Boltzmann equation helps describe how an ideal gas behaves.
This equation looks simple, but it's very complex to solve. It describes how the position and speed of gas particles change over time and space. The left side shows how the particles move and are affected by forces. The right side shows what happens when particles collide with each other.
Boltzmann tried for many years to prove the second law of thermodynamics using this equation. He used an idea called "molecular chaos." This idea assumes that particle collisions are random. However, this assumption makes it seem like time only moves forward, which caused problems in his proof.
The Second Law of Thermodynamics and Disorder
Boltzmann believed that the second law of thermodynamics is about disorder. This law says that systems naturally tend to become more disordered.
He imagined gas molecules as tiny billiard balls bouncing around in a box. With each collision, the molecules become more mixed up and disordered. This leads to a state of maximum disorder, which is also the state of maximum entropy.
Boltzmann argued that disordered states are the most likely to happen. There are many more ways for a system to be disordered than ordered. So, a system will almost always move towards a state of maximum disorder.
He showed that the second law of thermodynamics is a statistical fact. It's like shuffling a deck of cards. If you shuffle them enough, they will become completely mixed up. It's very unlikely they will go back to their original order by chance. Similarly, molecules in a system tend to move from less probable, ordered states to more probable, disordered states.
Works
Awards and Honours
In 1885, Boltzmann became a member of the Imperial Austrian Academy of Sciences. In 1887, he became the President of the University of Graz. He was also chosen as a member of the Royal Swedish Academy of Sciences in 1888. In 1899, he became a Foreign Member of the Royal Society. Many things are named after him to honor his work.
See also
 In Spanish: Ludwig Boltzmann para niños
In Spanish: Ludwig Boltzmann para niños
- Thermodynamics
- Boltzmann brain
 | Audre Lorde |
 | John Berry Meachum |
 | Ferdinand Lee Barnett |