August Kekulé facts for kids
Quick facts for kids
August Kekulé
|
|
|---|---|
August Kekulé
|
|
| Born |
Friedrich August Kekulé
7 September 1829 |
| Died | 13 July 1896 (aged 66) |
| Nationality | German |
| Alma mater | University of Giessen |
| Known for | Theory of chemical structure Tetravalence of carbon Structure of benzene |
| Awards | Copley Medal (1885) |
| Scientific career | |
| Institutions | University of Heidelberg University of Ghent University of Bonn |
| Thesis | Ueber die Amyloxydschwefelsäure und einige ihrer Salze (1852) |
| Academic advisors | Justus von Liebig |
| Doctoral students | Jacobus Henricus van 't Hoff Hermann Emil Fischer Adolf von Baeyer Richard Anschütz |
| Influences | Alexander Williamson Charles Gerhardt Auguste Laurent William Odling Charles Adolphe Wurtz |
| Influenced | Albert Ladenburg |
Friedrich August Kekulé, also known as August Kekulé, was an important German chemist. He lived from 1829 to 1896. From the 1850s until his death, Kekulé was one of the most famous chemists in Europe. He was especially known for his work in theoretical chemistry.
Kekulé is considered the main founder of the idea of chemical structure. This idea helps us understand how atoms are connected in molecules. He is also famous for figuring out the structure of benzene, a very important chemical.
Contents
About August Kekulé's Name
Kekulé was always known as August Kekulé. He never used his first name, Friedrich. In 1895, he was honored by the Kaiser (a German emperor). After this, he added "von Stradonitz" to his name. This part of the name came from his family's old property.
Kekulé's Early Life and Studies
August Kekulé was born in Darmstadt, Germany. His father worked for the government. In 1847, after finishing high school, Kekulé went to the University of Giessen. He first planned to study architecture.
However, he attended lectures by the famous chemist Justus von Liebig. These lectures changed his mind. Kekulé decided to study chemistry instead. After four years of study, he worked in Paris, Switzerland, and London. In London, he was greatly influenced by chemist Alexander Williamson. He earned his doctoral degree in 1852.
Understanding Chemical Structures
In 1856, Kekulé started teaching at the University of Heidelberg. Later, he became a professor at the University of Ghent and then at the University of Bonn. He stayed in Bonn for the rest of his career.
Kekulé built on ideas from other chemists. He became the main person to develop the theory of chemical structure. This theory explains how atoms are arranged in molecules. A key part of his theory was that carbon atoms can connect to four other atoms. He also showed that carbon atoms can link together in chains.
This new way of thinking brought great clarity to organic chemistry. It helped chemists understand and create new molecules. Because of this, the field of organic chemistry grew very quickly.
Chemists in Kekulé's time did not have tools like X-ray machines. They had to figure out structures based on how chemicals reacted. Kekulé's idea of "affinity units" (now called "valences" or "bonds") helped them connect atoms. His work laid the foundation for how we understand molecules today.
The Structure of Benzene
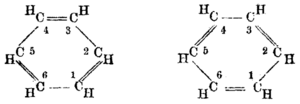
Kekulé's most famous discovery was the structure of benzene. In 1865, he published a paper suggesting its structure. He proposed that benzene has a ring of six carbon atoms. These carbon atoms are connected by alternating single and double bonds.
Before Kekulé, chemists knew benzene's formula but not its shape. It was hard to figure out because it had many bonds. By 1865, more clues were available. Kekulé noticed that all six carbon atoms in benzene seemed the same. This meant that no matter where you attached something to the ring, you got only one type of product.
For molecules related to benzene with two added parts, three different forms were found. Kekulé suggested these forms had the parts separated by one, two, or three carbon bonds. These are now called ortho, meta, and para isomers.
Some chemists pointed out a problem with his first idea. It seemed to suggest two types of ortho structures. To fix this, Kekulé updated his idea in 1872. He suggested that the bonds in benzene are constantly changing positions. This makes all six carbon-carbon bonds equal. Later, in 1928, Linus Pauling explained this better with the idea of resonance.
Kekulé's Dream About Benzene
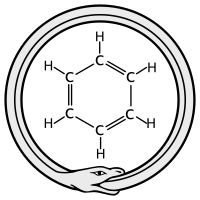
The discovery of benzene's structure was very important. In 1890, the German Chemical Society celebrated the 25th anniversary of Kekulé's benzene paper. At this event, Kekulé shared a famous story.
He said he discovered the ring shape of benzene in a dream. In his dream, he saw a snake biting its own tail. This ancient symbol is called the ouroboros. This story shows how imagination can help scientists.
Some historians think this dream story might not be entirely true. A similar funny drawing of benzene with monkeys had appeared earlier. Some believe Kekulé's story was a playful response to that drawing. Regardless, the story is now a famous part of chemistry history.
He also told another story about a dream that led to his theory of structure. He claimed this happened while riding a horse-drawn bus in London. In this dream, he saw atoms dancing and linking together.
Honors and Legacy
In 1895, Kaiser Wilhelm II of Germany honored Kekulé. He was given the right to add "von Stradonitz" to his name. This made his full name Friedrich August Kekule von Stradonitz.
Kekulé's influence was huge. Three of the first five Nobel Prizes in Chemistry were won by his former students. These students were van 't Hoff (1901), Fischer (1902), and Baeyer (1905).
A large statue of Kekulé was put up in 1903. It stands in front of the old Chemical Institute at the University of Bonn. Students sometimes decorate his statue for fun holidays.
See Also
 In Spanish: August Kekulé para niños
In Spanish: August Kekulé para niños
- Non-Kekulé molecule
- Skeletal formula
- Kekulé Program
- Auguste Laurent