Molar concentration facts for kids
Molar concentration, also called molarity, is a way to measure how much of a substance is mixed into a solution. Think of it like how much sugar is in your lemonade! It's a key idea in chemistry.
Molarity tells us the amount of a specific substance (called the "solute") that is dissolved in a certain volume of the total solution.
What is Molar Concentration?
In chemistry, we use a special formula to find molar concentration, which is often written as 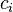 .
.
The formula looks like this:
Let's break it down:
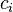 is the molar concentration (or molarity).
is the molar concentration (or molarity).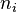 is the amount of substance. This is usually measured in moles. A mole is just a way to count a very large number of tiny particles, like atoms or molecules. It's like saying a "dozen" for 12 eggs, but a mole is for a much, much bigger number of particles!
is the amount of substance. This is usually measured in moles. A mole is just a way to count a very large number of tiny particles, like atoms or molecules. It's like saying a "dozen" for 12 eggs, but a mole is for a much, much bigger number of particles!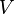 is the volume of the entire mixture or solution.
is the volume of the entire mixture or solution.
Solution Volume Explained
It's important to remember that the volume 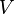 in the formula refers to the total volume of the solution, not just the volume of the solvent. The solvent is the liquid that dissolves the substance (like water in lemonade).
in the formula refers to the total volume of the solution, not just the volume of the solvent. The solvent is the liquid that dissolves the substance (like water in lemonade).
When you dissolve a substance in a solvent, the total volume of the liquid can change a little. For example, if you add sugar to one litre of water, the final volume of the sugary water might be slightly more or slightly less than one litre. This is why we always use the final volume of the solution.
Why is Molarity Important?
Molarity is super useful for scientists and chemists. It helps them:
- Know the exact strength of a chemical solution.
- Mix chemicals correctly for experiments or to make new products.
- Understand how much of a substance is present in things like blood tests or environmental samples.
See also

- In Spanish: Molaridad para niños