Nitrile facts for kids
A nitrile is a special type of chemical compound. It has a unique part called a functional group where a carbon atom is strongly connected to a nitrogen atom. This connection looks like C≡N. Many nitriles have a smell that reminds people of fruit. However, some nitriles can be harmful if not handled carefully.
Contents
What is a Nitrile?
A nitrile is a molecule that contains a specific chemical group known as the "cyano group." This group is made of one carbon atom and one nitrogen atom joined together by a triple bond. This triple bond means the atoms share three pairs of electrons, making the connection very strong. The cyano group is what gives nitriles their special properties.
The Cyano Functional Group
A functional group is like a special building block within a larger molecule. It's a small group of atoms that always acts in a similar way, no matter what other atoms are in the molecule. For nitriles, the functional group is the C≡N (cyano) part. This group helps scientists understand how a nitrile will react with other chemicals.
Properties of Nitriles
Nitriles have different properties depending on their exact structure. Some nitriles are liquids, while others are solids. As mentioned, many have a distinct fruity smell. They are used in various ways, from making plastics and rubber to creating medicines.
Uses of Nitriles
Nitriles are important in many industries. For example, they are used to make:
- Synthetic rubber: This type of rubber is used in gloves, hoses, and seals because it's strong and resistant to oils.
- Plastics: Some plastics, like those used in car parts or pipes, contain nitriles.
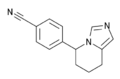
- Medicines: Certain medicines have nitrile groups in their structure. For instance, fadrozole is a medicine that helps treat breast cancer, and it contains a nitrile group.
Images for kids
See also
 In Spanish: Nitrilo para niños
In Spanish: Nitrilo para niños
 | Mary Eliza Mahoney |
 | Susie King Taylor |
 | Ida Gray |
 | Eliza Ann Grier |