Antimicrobial peptides facts for kids
Antimicrobial peptides (AMPs) are like tiny defenders found in all living things, from bacteria to humans. They are a key part of our body's first line of defense against germs. These special peptides (which are small proteins) are very strong and can fight many types of harmful invaders. They can kill different kinds of bacteria, viruses, and fungi. They can even target some cancer cells!
Unlike many regular medicines, AMPs often work by breaking down the outer layer of germs, like popping a balloon. They can also form tiny holes in these layers, or even help our immune system fight off infections better.
Contents
What They Are Like
Antimicrobial peptides are a very diverse group of molecules. They are usually made of 12 to 50 tiny building blocks called amino acids. These peptides often have two or more positively charged parts and a lot of parts that don't like water (hydrophobic parts).
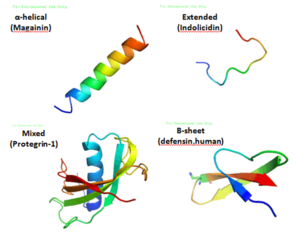
They can have different shapes, like:
- Spiral shapes (called α-helical)
- Folded sheet shapes (called β-stranded)
- Loop shapes (called β-hairpin)
- Stretched out shapes
Many of these peptides are floppy until they touch a germ's outer layer. Then, they fold into their final shape. They have one side that likes water and another side that doesn't. This special design helps them stick to and get inside the outer layer of germs. This ability to connect with germ layers is a key feature of AMPs. They can kill germs in many ways, from breaking their outer layer to messing with their inner workings.
Here are some examples of different types of AMPs:
| Type | What they are like | Examples |
|---|---|---|
| Anionic peptides | Rich in glutamic and aspartic acids | Maximin H5 (from amphibians), dermcidin (from humans) |
| Linear cationic α-helical peptides | Don't have cysteine | Cecropins (from insects), Magainin (from amphibians), CAP18 (from rabbits), LL37 (from humans) |
| Cationic peptides with specific amino acids | Rich in proline, arginine, phenylalanine, glycine, tryptophan | Abaecin and drosocin (from insects), prophenin (from pigs), indolicidin (from cattle) |
| Peptides with disulfide bonds | Have 1 to 3 bonds |
|
How They Work
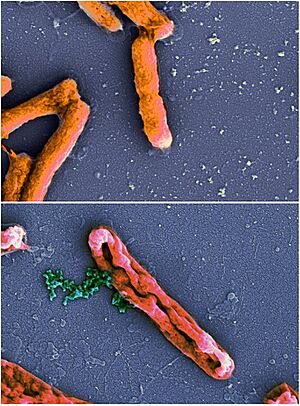
Antimicrobial peptides kill germs in many different ways. Some AMPs can kill both bacteria and fungi. For example, psoriasin can kill E. coli bacteria and some fungi.
Often, AMPs target the outer layer of a germ. But they can also mess with a germ's:
- DNA (its genetic code)
- Protein making
- Protein folding
- Cell wall building
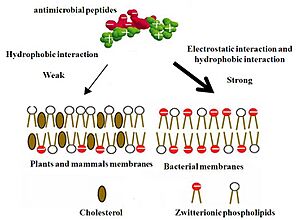
The first step is usually the AMP sticking to the germ. This happens because most germ surfaces are negatively charged, and AMPs are positively charged. Or, the AMP's water-hating parts stick to the germ's outer layer.
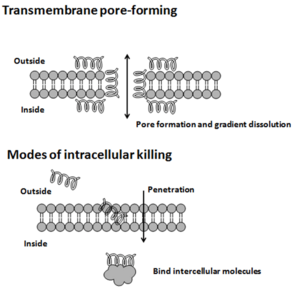
Once attached, AMPs can poke holes in the germ's outer layer using different methods, like the 'barrel-stave' or 'carpet' models. Or, they might get inside the germ and bind to important molecules that the germ needs to live. This can stop the germ from building its cell wall, change its outer layer, or stop it from making DNA, RNA, or proteins.
Unlike many regular medicines, AMPs usually kill germs directly, rather than just stopping them from growing. Scientists often measure how well an AMP works by finding the lowest amount needed to stop a germ from growing. This is called the Minimal Inhibitory Concentration (MIC).
AMPs can do many things, including fighting:
- Gram-positive bacteria
- Gram-negative bacteria
- Fungi
- Viruses
- Parasites
- Cancer cells
Studies show that for AMPs to fight Gram-negative bacteria, they often need to be very good at both liking and hating water (amphipathicity) and have a strong positive charge.
Boosting Immunity
AMPs don't just kill germs directly. They also help our immune system in many ways. They can:
- Change how our body's genes work
- Act like chemical signals (chemokines) that attract immune cells
- Stop harmful inflammation
- Help wounds heal
- Change how immune cells (like dendritic cells) respond
Studies in animals show that these peptides are very important for both stopping and clearing up infections. Some peptides first found as "antimicrobial peptides" are now known to have even more important roles in the body. For example, a peptide called Dusquetide is being studied to see if it can help repair damage from cancer radiation treatment.
How They Break Down Germs
Antimicrobial peptides usually have a positive charge. This helps them stick to the negatively charged outer layers of bacteria and cancer cells.
The way AMPs work can be put into two main groups:
- Membrane-breaking AMPs: These break down the outer layer of the germ.
- Non-membrane-breaking AMPs: These get inside the germ and mess with its internal parts.
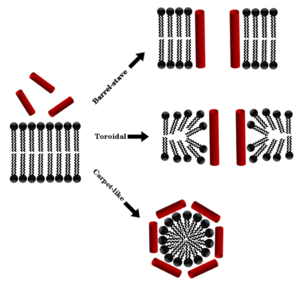
Here are four ways membrane-breaking AMPs can disrupt germ outer layers:
- Barrel-stave model: Imagine the AMPs forming a tube or "barrel" through the germ's outer layer. This hole lets things leak out, killing the germ.
- Carpet model: The AMPs cover the germ's outer layer like a dense carpet. This covering makes the layer leaky, stopping the germ from working properly.
- Toroidal model: The AMPs work with the germ's outer layer to form ring-shaped structures. These rings can pinch off parts of the outer layer, forming small bubbles and breaking the layer apart.
- Disordered toroidal-pore model: Similar to the toroidal model, but the AMPs are more flexible and don't form a stable ring. They still create a hole that breaks the outer layer and kills the germ.
Scientists use many methods to figure out how AMPs work. For example, special types of solid-state NMR and X-ray crystallography can show exactly how AMPs break down germ outer layers. Computer simulations (Molecular Dynamics) also help scientists understand how AMPs interact with these layers.
Here are some methods used to study AMPs:
| Methods | What they are used for |
|---|---|
| Microscopy | To see how AMPs affect germ cells |
| Atomic emission spectroscopy | To detect if potassium leaks out of bacteria (a sign their outer layer is broken) |
| Fluorescent dyes | To see if AMPs make outer layers leaky |
| Ion channel formation | To check if AMPs form stable holes |
| Circular dichroism | To measure the shape and direction of AMPs when they stick to outer layers |
| Dual polarization interferometry | To study different ways AMPs work |
| Solid-state NMR spectroscopy | To see the shape, direction, and how deep AMPs go into outer layers |
| Neutron and X-ray diffraction | To measure patterns of holes AMPs make in outer layers |
| Molecular dynamics simulations | To study how AMPs behave and interact with outer layers |
| Mass spectrometry | To see how germs change their proteins when exposed to AMPs |
AMPs as Medicines
Antimicrobial peptides are used as medicines, but usually only as shots or on the skin. This is because they don't last very long in the body.
As of early 2018, some AMPs in use were:
- Bacitracin for pneumonia (used on skin)
- Boceprevir for Hepatitis C (taken by mouth)
- Daptomycin for bacterial infections (given by IV)
- Enfuvirtide for HIV (shot under the skin)
- Vancomycin for bacterial infections (given by IV)
- Guavanin 2 for bacterial infections (both Gram-positive and Gram-negative)
More Than Just Antibacterial
AMPs do more than just kill bacteria and fungi. They can also fight viruses, help with cancer, and even play roles in the nervous system. Because of this, some people think AMPs should be called "Host-defense peptides" to show all the different ways they help protect us.
Fighting Cancer
Some AMPs, like certain cecropins, can fight cancer cells. These are called anticancer peptides (ACPs). For example, a defensin from fruit flies can stop tumors from growing. It's thought to stick to cancer cells because their outer layers are different from healthy cells.
Fighting Biofilms
Cecropin A can destroy both free-floating bacteria and bacteria that form sticky layers called biofilms. Biofilms are like protective shields that make bacteria harder to kill. Cecropin A can break down these biofilms, especially when combined with other medicines. It works by making the outer layer of bacteria leaky and messing with their ability to pump out medicines.
Other Research
Scientists are also looking for new AMPs from different sources, like bacteria, fish, shellfish, and even animals like echidnas.
Why They Target Bad Cells
AMPs are amazing because they usually attack harmful bacteria and cancer cells, but leave our healthy cells alone. This is called "selectivity."
Here's why they are selective:
- Charge: The outer layers of bacteria are usually more negatively charged than our healthy cells. Since AMPs are positively charged, they are more attracted to bacteria.
- Cholesterol: Our healthy cells have cholesterol in their outer layers, which makes them stronger and harder for AMPs to break. Bacteria don't have cholesterol.
- Electrical Charge: The inside of bacterial cells is more negatively charged than the outside. This electrical difference helps positively charged AMPs get inside and attack.
- Saltiness: High salt levels can reduce how well AMPs work. This also helps protect our cells, as our body fluids have a certain saltiness.
How Selectivity Works
Bacterial outer layers are rich in negatively charged fats. In contrast, the outer part of our healthy cells mainly has fats without a charge. The negatively charged fats in our cells are mostly hidden on the inside. So, AMPs are more likely to stick to and break down the negatively charged outer layers of bacteria.
Scientists are trying to make AMPs even more selective. They are changing things like the AMP's charge, shape, and how much it dislikes water. They are also adding special amino acids to make AMPs less harmful to healthy cells.
How Bacteria Resist AMPs
Bacteria can develop ways to fight back against AMPs. This is called Antimicrobial resistance.
- Some bacteria change the charge of their outer layer to be less negative, so AMPs are less attracted to them.
- Some bacteria produce a protective slime layer that stops AMPs from reaching them.
- Salmonella bacteria can make their outer layer tougher, making it harder for AMPs to poke holes.
- Some bacteria can pull AMPs inside their cells and break them down.
- Bacteria can also pump AMPs out of their cells.
- Some bacteria produce enzymes that destroy AMPs.
- Gram-negative bacteria can release tiny bubbles from their outer layer that trap AMPs, keeping them away from the main cell.
There's a concern that if we use AMPs as medicines too much, bacteria might become resistant to them more quickly. This resistance could then affect how well our natural AMPs work in our bodies.
One idea to get around this resistance is the "Trojan Horse" approach. This involves attaching AMPs to molecules that bacteria need, like iron. The bacteria then "swallow" the AMP along with the iron, bringing the killer inside.
Examples
Antimicrobial peptides are found in many different living things, including:
- Bacteria (like bacteriocins)
- Fungi (like plectasin)
- Insects and arthropods (like cecropin and melittin)
- Amphibians (like magainin and dermaseptin)
- Birds (like avian defensins)
- Mammals (like cathelicidins and defensins)
Scientists are also creating artificial AMPs because natural ones can be expensive to produce. For example, nisin is an AMP commonly used as a food preservative.
Finding AMPs with Computers
There are special online databases that collect information about antimicrobial peptides. The Antimicrobial Peptide Database (APD) is a very important one. Other databases include ADAM, BioPD, CAMP, DBAASP, DRAMP, and LAMP.
These databases help scientists:
- Find AMPs
- Study their features
- Predict new AMPs
- Understand how they work against different germs
For example, dbAMP is an online tool that helps explore AMPs and their activities, shapes, and how they interact with other proteins.
See Also
 In Spanish: Péptido antimicrobiano para niños
In Spanish: Péptido antimicrobiano para niños
- Aurein
- Bacteriocin
- Cathelicidin
- Copsin
- Diptericin
- Peripheral membrane proteins
- Virtual colony count
 | Selma Burke |
 | Pauline Powell Burns |
 | Frederick J. Brown |
 | Robert Blackburn |