Bullvalene facts for kids
Bullvalene is a fascinating chemical compound. It's a type of hydrocarbon, which means it's made up of only hydrogen and carbon atoms. Its chemical formula is C10H10, meaning it has 10 carbon atoms and 10 hydrogen atoms. What makes Bullvalene truly special is that it's a molecule with no permanent structure. It's constantly changing its shape!
Contents
What Makes Bullvalene Unique?
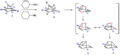
Bullvalene is known for its amazing ability to rearrange its atoms very quickly. Imagine a shape-shifting toy that changes its form many times per second – that's a bit like Bullvalene! This rapid change happens because its chemical bonds are always breaking and reforming.
How Does Bullvalene Change Shape?
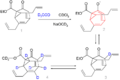
The atoms in Bullvalene are always moving around in a process called "fluxionality." This means that at room temperature, all the carbon and hydrogen atoms in the molecule can swap places. It's like a chemical dance where every atom can end up in any position. This happens through a special kind of chemical reaction called a Cope rearrangement.
The Cope Rearrangement Explained
A Cope rearrangement is a chemical reaction where atoms within a molecule rearrange themselves without needing any other chemicals to be added. In Bullvalene, this rearrangement happens over and over again. It's so fast that if you tried to take a picture of its structure, you'd get a blurry image because it's never still! This makes Bullvalene a very interesting molecule for scientists to study.
Discovering Bullvalene
Bullvalene was first made by scientists in 1963. Its unique properties quickly made it famous in the world of chemistry. Scientists were excited to find a molecule that could change its structure so rapidly and without much energy.
Why is Bullvalene Important?
Studying Bullvalene helps scientists understand how molecules can change their shapes and how chemical bonds work. This knowledge can be useful in many areas, like designing new materials or understanding complex biological processes where molecules also change their forms. It's a great example of how chemistry can be full of surprises and constant motion!
Similar Molecules
While Bullvalene is famous for its shape-shifting, other compounds share its exact chemical formula (C10H10). These are called "isomers." Some examples include:
- Basketene
- Cyclodecapentaene
- Dialin
- Divinylbenzene
- Diisopropenyldiacetylene
- Pentaprismane (also known as [5]Prismane)
These molecules have the same number of carbon and hydrogen atoms as Bullvalene, but their atoms are arranged differently, giving them different shapes and properties.
Images for kids