Chemical cell facts for kids
A chemical cell is a special device that turns chemical energy into electrical energy. Think of it as a tiny power plant! Most batteries you use every day are chemical cells. Inside these cells, a chemical reaction happens. This reaction creates a flow of electric current, which is what powers your devices.
There are two main kinds of batteries you'll find: those you can use only once, and those you can recharge many times.
A battery that isn't rechargeable works until all its special chemicals are used up. Once that happens, it can't make electricity anymore. You might call these "single-use" or "disposable" batteries. After they're empty, you just throw them away.
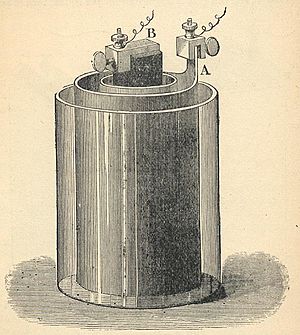
A rechargeable battery is different. When it runs out of power, you can "recharge" it. This means you send electric current back through it. This process makes the chemicals inside ready to work again. Then, you can use the battery to make more electricity! A French scientist named Gaston Plante invented these handy rechargeable batteries way back in 1859.
Batteries come in all sorts of shapes and sizes. You can find tiny ones for toys or cameras. There are also bigger ones used in cars, and even huge batteries for other purposes.
Contents
How Batteries Make Electricity
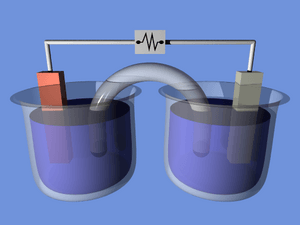
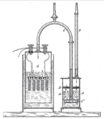
Inside every chemical cell, there are two different metal parts, often called electrodes. These electrodes are placed in a special liquid or paste called an electrolyte. The chemicals in the electrolyte react with the metals. This reaction causes tiny particles called electrons to move from one electrode to the other.
When electrons move, they create an electric current. This current can then flow through a wire to power your device. It's like a tiny, controlled lightning bolt! The chemical reaction keeps happening until one of the chemicals runs out.
Types of Chemical Cells
There are many different types of chemical cells, each designed for specific uses. Here are a few examples:
Simple Chemical Cells
A simple chemical cell is like the very first battery ever made. It usually has two different metals, like copper and zinc. These metals are put into an acid solution. The acid reacts with the metals, causing electrons to flow. This creates a small amount of electricity. It's a great way to understand the basic idea of how batteries work.
Dry Cells
Most of the batteries you use in flashlights or remote controls are "dry cells." They are called dry cells because they don't have a liquid electrolyte that can spill. Instead, they use a moist paste. This makes them much safer and easier to use. They are very common because they are cheap to make and work well for many everyday items.
Wet Cells
"Wet cells" use a liquid electrolyte. The most common example is the battery in a car. Car batteries use lead plates and sulfuric acid. These batteries can provide a lot of power, especially for starting an engine. They are often rechargeable, which is important for cars.
Fuel Cells
Fuel cells are a bit different from other batteries. They don't run out of chemicals in the same way. Instead, they use a continuous supply of fuel, like hydrogen gas, and oxygen from the air. As long as they have fuel, they can keep producing electricity. They are very efficient and produce very little pollution. This makes them exciting for future energy needs.
Images for kids
 | Bayard Rustin |
 | Jeannette Carter |
 | Jeremiah A. Brown |