Delocalized electron facts for kids
A delocalized electron is an electron that isn't stuck to just one atom or one specific bond in a molecule, ion, or solid metal. Think of it like a tiny, super-fast runner that can move freely across a larger area instead of being tied to a single spot. The idea of "delocalization" simply means that something, like an electron, is spread out over a bigger space. This concept is really important in chemistry because it helps explain why many substances behave the way they do.
Contents
What Are Delocalized Electrons?
Normally, electrons in atoms are found in specific orbits around the nucleus. When atoms join together to form molecules, they often share electrons in what are called covalent bonds. In a typical covalent bond, two atoms share a pair of electrons, and these electrons stay mostly between those two atoms.
However, some molecules and materials have a special arrangement where electrons are not held tightly by just two atoms. Instead, they are shared among three or more atoms. These are the delocalized electrons. They can move around a larger region, almost like a cloud of charge spread over several atoms.
Where Do We Find Them?
Delocalized electrons are found in several interesting types of chemical structures. They give these substances unique properties.
Benzene: A Special Ring
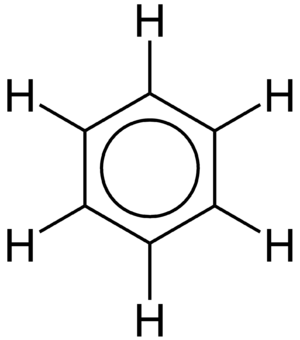
One of the most famous examples of delocalized electrons is in a molecule called benzene. Benzene has a ring shape made of six carbon atoms. Each carbon atom is also bonded to one hydrogen atom. If you look at the bonds in benzene, you might expect to see alternating single and double bonds.
But in reality, all the carbon-carbon bonds in benzene are exactly the same length and strength. This is because the electrons that would normally form the double bonds are actually delocalized. They are spread out evenly over the entire six-carbon ring. This makes benzene very stable and less reactive than other molecules with double bonds.
Metals: Free-Moving Electrons
Another great example of delocalized electrons is found in metals. Metals like copper, iron, or aluminum are made of many metal atoms packed closely together. The outer electrons from each metal atom are not held by any single atom. Instead, they form a "sea" of delocalized electrons that can move freely throughout the entire metal structure.
This "sea" of electrons is why metals are such good conductors of electricity and heat. The electrons can easily flow from one end of the metal to the other, carrying energy with them. It also explains why metals are shiny and can be bent into different shapes without breaking.
Other Examples: Conjugated Systems
Delocalized electrons also appear in what are called conjugated systems. These are molecules that have alternating single and double (or triple) bonds. For example, in a molecule like butadiene, which has four carbon atoms, the electrons in the double bonds can spread out over the single bonds in between.
This delocalization can affect the color of substances. Many dyes and pigments have conjugated systems with delocalized electrons. When light hits these molecules, the delocalized electrons can absorb certain colors of light, making the substance appear colorful.
Why Are They Important?
The presence of delocalized electrons has a big impact on the properties of materials.
Stability and Strength
When electrons are delocalized, they are spread out over a larger volume. This spreading out makes the molecule or material more stable. It's like having more space to move around; the electrons are less crowded and have lower energy. This extra stability is why molecules like benzene are so strong and unreactive.
Electrical Conductivity
As mentioned with metals, delocalized electrons are essential for electrical conductivity. Since these electrons are not tied to specific atoms, they can easily move when an electric voltage is applied. This movement of electrons is what we call an electric current. Materials with delocalized electrons are often good conductors.
They also play a role in the conductivity of some plastics, known as conductive polymers. These special plastics have delocalized electrons that allow them to conduct electricity, opening up new possibilities for flexible electronics.
See also
 In Spanish: Deslocalización electrónica para niños
In Spanish: Deslocalización electrónica para niños

