Equilibrium constant facts for kids
Equilibrium constant is a special number that helps us understand what happens when a chemical reaction reaches a balanced state called chemical equilibrium. Imagine a seesaw that's perfectly still – that's like a reaction at equilibrium!
This constant tells us if a reaction will have more products (the new things made) or more reactants (the starting materials) when it's balanced. It can also help scientists know if a reaction has already reached this balanced state.
There are different kinds of equilibrium constants. For example, Dissociation constants are used to describe how much a substance breaks apart into smaller pieces.
Contents
What is an Equilibrium Constant?
When chemicals react, they don't always go in just one direction. Often, the products can react to turn back into the original reactants. When the speed of the forward reaction (reactants to products) becomes equal to the speed of the backward reaction (products to reactants), the system reaches a chemical equilibrium. At this point, the amounts of reactants and products stay constant.
The equilibrium constant, often written as K, is a ratio that compares the amounts of products to the amounts of reactants at equilibrium.
For a general reaction like: A + B ⇌ C + D
The equilibrium constant is usually written like this: ![K_c=\frac{{[C]} {[D]}} {{[A]} {[B]}}](/images/math/2/1/c/21c83f137e223fec2e3eefbd4b6a0568.png)
Here, the square brackets, like `[A]`, mean the concentration of that chemical (how much of it is in a certain space). The products are always on top (numerator) and the reactants are on the bottom (denominator).
Think of it this way: In a balanced reaction, atoms are constantly combining and breaking apart. For a reaction to happen, all the necessary parts need to be in the same place at the same time. If there's a lot of a certain chemical, it's more likely to bump into other chemicals and react.
So, the concentrations of all the chemicals needed for one side of the reaction are multiplied together. The same is done for the other side. The ratio between these two numbers shows how much of each side will be present when the reaction is balanced.
- If the equilibrium constant (K) is a large number (much bigger than 1), it means there will be more products than reactants at equilibrium. The reaction "prefers" to make products.
- If K is a small number (much smaller than 1), it means there will be more reactants than products at equilibrium. The reaction "prefers" to stay as reactants.
- If K is close to 1, it means there are similar amounts of products and reactants at equilibrium.
Scientists do experiments to measure the equilibrium constant for many different reactions.
Different Types of Equilibrium Constants
Sometimes, instead of using concentrations, scientists use partial pressures for reactions involving gases. This gives us another type of equilibrium constant called Kp.
The relationship between Kc (using concentrations) and Kp (using pressures) depends on how many gas molecules are on each side of the balanced reaction.
For a reaction where gases are involved: 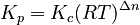
Here:
- `R` is the universal gas constant.
- `T` is the temperature in Kelvin.
- `Δn` (delta n) is the total number of gas molecules on the product side minus the total number of gas molecules on the reactant side.
This formula helps scientists convert between these two types of equilibrium constants when working with gases.
Images for kids
See also
 In Spanish: Constante de equilibrio para niños
In Spanish: Constante de equilibrio para niños