Flame ionization detector facts for kids
A Flame Ionization Detector (often called an FID) is a special tool used in a science method called gas chromatography. It's really good at finding and measuring tiny amounts of organic compounds. These are compounds that contain carbon, like the ones found in living things. The first FID was invented in Australia in 1957.
Contents
What is a Flame Ionization Detector?
An FID helps scientists detect different substances after they have been separated by a machine called a gas chromatograph. Imagine a gas chromatograph as a super-sniffer that separates a mixture of gases. The FID is like the final sensor that tells you what gases are present and how much of each there is.
How Does a Flame Ionization Detector Work?
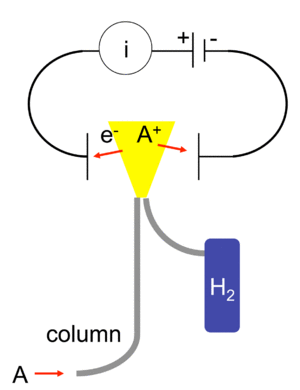
When the gases leave the gas chromatograph, they enter the FID. Inside the FID, there's a small flame, usually made from burning hydrogen and oxygen. As the gases pass through this flame, the organic compounds in them get very hot and break apart. When they break apart, they create tiny electrically charged pieces called ions and electrons.
These charged pieces are then collected by special plates inside the detector. When charged pieces are collected, they create a tiny electrical current. The more organic compounds that burn in the flame, the more ions and electrons are made, and the bigger the electrical current. This current is measured by a very sensitive device called a picoammeter. A larger current means there's more of that organic compound present. The currents are usually very, very small, about 10-12 amps.
What Can an FID Detect?
FIDs are mostly used for organic compounds because these compounds have many carbon atoms. Carbon atoms are good at turning into ions when they burn. Carbons that don't have oxygen attached to them (like in hydrocarbons) create the most ions. Other organic compounds, like alcohols (which have C-O-H bonds) or carbonyls (which have C=O bonds), still create ions but not as many.
Why FIDs are Useful
One great thing about FIDs is that they don't detect common impurities like water (H2O), carbon dioxide (CO2), sulfur dioxide (SO2), or noble gases. This is because these substances don't burn in the FID's flame and therefore don't create ions. This means that samples don't always need to be perfectly clean before being tested, which saves a lot of time and effort.
FIDs are also very sensitive. They can detect extremely small amounts of material, as little as 10-13 grams per second. They are also accurate over a very wide range of amounts, which means they can measure both tiny and larger quantities of compounds reliably.
What are the Challenges of Using an FID?
While FIDs are very useful, they do have some downsides:
- Sample Destruction: The biggest drawback is that the sample being tested is burned up in the flame. This means you can't use that same sample for any other tests afterwards.
- Fuel Needs: FIDs need a constant supply of hydrogen and oxygen gas to create the flame, which can be costly.
- Precision Equipment: To get very accurate results, the equipment needs to be set up and maintained very precisely.
See also
 In Spanish: Detector de ionización de llama para niños
In Spanish: Detector de ionización de llama para niños