Antibody facts for kids
Antibodies are special Y-shaped proteins found in your blood and other body fluids. They are also called immunoglobulins. Think of them as tiny defenders that help your body fight off invaders like bacteria and viruses. They are a super important part of your body's adaptive immune system, which learns to fight new threats.
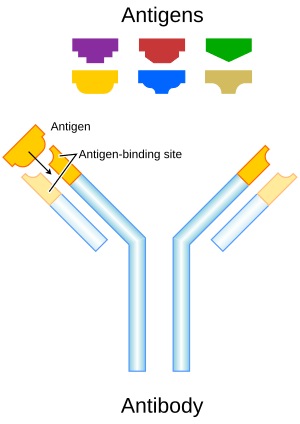
Each antibody is designed to find and stick to a specific part of an invader. This part is called an antigen. Imagine the tips of the Y-shape as a lock. This lock is made to fit only one specific key-like structure on an antigen. When the antibody's "lock" finds its matching "key" on an antigen, they bind together.
Once an antibody binds to an invader, it can do two main things. It can "tag" the microbe or an infected cell. This tag tells other parts of your immune system to come and destroy the invader. Or, the antibody can directly stop the invader from working, which is called neutralizing it. Making antibodies is a big job of your body's humoral immunity.
Every antibody is unique. They are made to attack only one type of antigen. For example, an antibody that fights the smallpox virus won't be able to fight the common cold virus. This is because their "locks" are different.
Even though all antibodies look similar, the small area at the tip of the Y-shape is very different in each one. This allows your body to create millions of different antibodies. Each one can bind to a different antigen. This huge variety helps your immune system recognize and fight against many different kinds of germs.
How Antibodies Are Made
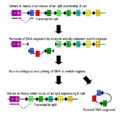
Your body needs to make millions of different antibodies to fight all the different germs out there. But your body only has a limited number of genes. So, how does it make so many different antibodies from a small number of genes?
Your body uses some clever tricks. It combines small pieces from different genes in many ways. This is like building different Lego models from the same set of bricks. After combining these pieces, tiny changes (called mutations) happen in the part of the gene that makes the antibody's binding site. These changes create even more variety.
Antibody Structure
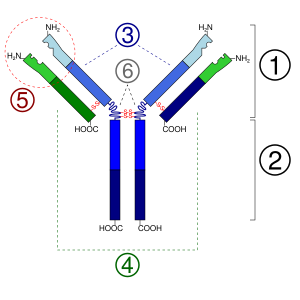
Antibodies are made of basic parts. Each antibody unit has two long chains called heavy chains and two shorter chains called light chains. There are different types of heavy chains, which group antibodies into different families. These different families help guide the right immune response for each type of invader.
The part of the antibody that binds to the antigen is at the very tip of the Y-shape. This area is called the hypervariable region because it changes a lot from one antibody to another. This is where the "lock" part of the antibody is located. The many different shapes of this region allow antibodies to bind to a huge variety of antigens.
- You can see how antibodies are used in tests like ELISA and ELISPOT .
Images for kids
-
Michael Heidelberger, a scientist who studied antibodies.
See also
 In Spanish: Anticuerpo para niños
In Spanish: Anticuerpo para niños