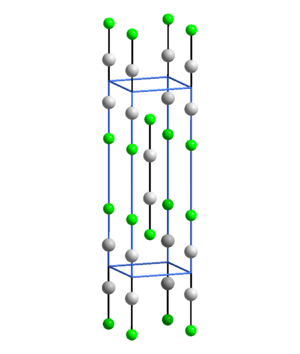
A diagram showing how the atoms are arranged in mercury(I) fluoride.
Mercury(I) fluoride, also known as mercurous fluoride, is a special chemical compound. Its chemical formula is Hg2F2. It contains mercury and fluoride ions. In this compound, the mercury atoms have a +1 electrical charge, which is called its oxidation state.
What is Mercury(I) Fluoride Like?
Mercury(I) fluoride is a light yellow solid. Imagine a pale yellow powder or crystal. It can turn into a gas, or "evaporate," if it gets very hot. This happens around 240 degrees Celsius. That's much hotter than boiling water! If you shine light on it, this compound changes color. It turns black when light hits it. Mercury(I) fluoride also reacts with water. When it mixes with water, it forms other substances. These include mercury metal, mercury(II) oxide, and hydrofluoric acid. It is important to know that this compound is dangerous. It should only be handled by trained scientists.
How is Mercury(I) Fluoride Made?
Scientists can make mercury(I) fluoride in a laboratory. They do this by mixing two other chemicals. These chemicals are mercury(I) carbonate and hydrofluoric acid. When these two react, they create mercury(I) fluoride.
Related Chemical Compounds






