Randomized controlled trial facts for kids
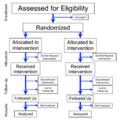
A randomized controlled trial is a special way to do a scientific experiment. It helps make sure the results are fair and accurate. This method is often used to test if medicines work well for different health problems.
In these trials, people who join are put into groups by chance. Each group gets a different treatment. At the end, scientists compare the results from all groups. For example, one group might get a placebo (a fake medicine). Another group would get the real medicine. A trial is called blinded if the people taking part, or even the doctors giving the medicine, do not know which group a patient is in. This helps prevent anyone's beliefs from affecting the results.
How These Studies Started
People have been doing controlled studies for a long time. In 1753, a doctor named James Lind published an important study. It showed that eating many lemons and oranges could treat a disease called scurvy.
Later, Ignaz Semmelweis, a doctor in Vienna, Austria, used "systematic controlled observation." He noticed that many women were getting childbed fever in hospitals. He figured out this was because doctors were not washing their hands.
By the late 1800s, people realized a problem. If you didn't put people into test groups randomly, the results might not be fair.
The First Randomized Trial
The term "randomized controlled trial" was created in the 1940s by Austin Bradford Hill. He led a study about treating tuberculosis with an antibiotic called Streptomycin. This study is now seen as the very first randomized controlled trial. It set the standard for how to test new medicines fairly.
Images for kids
See also
 In Spanish: Prueba controlada aleatorizada para niños
In Spanish: Prueba controlada aleatorizada para niños