Staggered conformation facts for kids
Imagine you have a tiny building block, like a molecule of ethane. This molecule has different parts that can spin around. A staggered conformation is a special way these parts can line up. It's like when you stand with your arms and legs spread out as much as possible, so you take up the most space and don't bump into anything.
In a staggered conformation, the parts of the molecule are as far away from each other as they can be. This makes the molecule very stable and balanced, almost like a perfectly symmetrical design.
Contents
What is a Staggered Conformation?
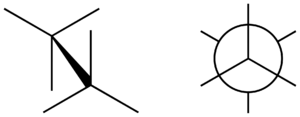
A staggered conformation happens when the atoms or groups of atoms on one part of a molecule are positioned exactly between the atoms on the other part. Think of it like looking down a train track: the rails on one side are perfectly in the middle of the rails on the other side.
How Atoms Line Up
For a simple molecule like ethane, which has two carbon atoms connected, the hydrogen atoms on one carbon are directly in the gaps between the hydrogen atoms on the other carbon. This arrangement helps the molecule avoid any crowding or pushing between its parts.
The Angle of Twist
When we talk about how much the parts of a molecule are twisted, we use something called a "torsion angle." In a perfectly staggered conformation, this angle is 60 degrees. This 60-degree twist is what makes everything line up so neatly, with each part having its own space.
Why is Staggered Conformation Important?
Molecules are always moving and twisting. But they prefer to be in the most comfortable and stable positions.
Energy and Stability
A staggered conformation is the most stable and lowest-energy arrangement for many molecules. This is because the atoms are spread out, so there's less "pushing" or "repulsion" between their electron clouds. Think of it like having enough personal space – it's more comfortable than being squished!
Staggered vs. Eclipsed
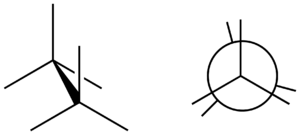
The opposite of a staggered conformation is an eclipsed conformation. In an eclipsed conformation, the atoms on one part of the molecule are directly in front of the atoms on the other part. This is like standing directly behind someone, so you can't see them.
Why Eclipsed is Less Stable
Eclipsed conformations have higher energy because the atoms are closer together. This causes more repulsion between their electron clouds, making the molecule less stable. Molecules usually try to avoid this position and quickly twist into a staggered shape.
How We See Conformations
Scientists use special drawings called "Newman projections" to show these different ways molecules can line up.
Newman Projections Explained
In a Newman projection, you look straight down a specific bond between two atoms (like the carbon-carbon bond in ethane). The front atom is shown as a dot, and the back atom is shown as a circle. The other atoms connected to them are drawn as lines coming out from the dot or the circle.
Seeing Staggered in a Drawing
When you draw a staggered conformation using a Newman projection, you can clearly see the lines from the front atom fitting perfectly into the spaces between the lines from the back atom. This visual helps scientists understand how molecules behave.
 | Kyle Baker |
 | Joseph Yoakum |
 | Laura Wheeler Waring |
 | Henry Ossawa Tanner |