Stoichiometry facts for kids
Stoichiometry (say: stoy-key-AH-muh-tree) is a part of science that helps us understand and measure the amounts of things involved in chemical reactions. Think of it like a recipe for chemistry! Just as a recipe tells you how much flour and sugar you need to bake a cake, stoichiometry tells chemists how much of each chemical they need to make a new substance, and how much of that new substance they can expect to get.
It's super useful for predicting how much "stuff" will be made or used up in a chemical reaction. This helps scientists and engineers avoid waste and make sure they have enough ingredients.
Contents
What is Stoichiometry?
Stoichiometry is all about the numbers in chemistry. It uses balanced chemical equations to figure out the exact amounts of substances that react and are produced. It's like being a detective for atoms and molecules, making sure everything adds up perfectly.
Why is it Important?
- Making New Things: If you want to make a new medicine or a new plastic, stoichiometry helps you know exactly how much of each starting material you need.
- Saving Money: By knowing the right amounts, companies don't waste expensive chemicals.
- Safety: In some reactions, using too much of one chemical can be dangerous. Stoichiometry helps keep things safe.
- Understanding Nature: It helps us understand how chemical processes work in nature, like how plants grow or how our bodies digest food.
How Does it Work?
Stoichiometry relies on a few important rules of chemistry:
Chemical Equations as Recipes
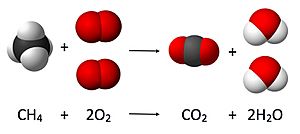
Chemical reactions are written as equations, like this: 2H₂ + O₂ → 2H₂O
- The numbers in front of the chemical formulas (like the "2" in 2H₂) are called coefficients. They tell us how many molecules of each substance are involved.
- The arrows (→) mean "yields" or "produces."
- This equation tells us that two molecules of hydrogen gas (H₂) react with one molecule of oxygen gas (O₂) to produce two molecules of water (H₂O).
Balancing the Equation
Before you can use stoichiometry, the chemical equation must be balanced. This means that the number of atoms of each element must be the same on both sides of the equation. It's like making sure you have the same number of ingredients before and after you cook!
This idea comes from the law of conservation of mass. This law says that matter cannot be created or destroyed in a chemical reaction. So, the total mass of the chemicals you start with must equal the total mass of the chemicals you end up with.
Using Moles to Count
Atoms and molecules are tiny, so chemists use a special unit called the mole to count them. One mole is a very large number (about 6.022 x 10²³ particles!). It's like using a "dozen" to count eggs, but for atoms.
By using moles, chemists can easily compare the amounts of different substances in a reaction. For example, in the water equation (2H₂ + O₂ → 2H₂O), it also means that 2 moles of hydrogen react with 1 mole of oxygen to make 2 moles of water.
Calculations in Stoichiometry
Once you have a balanced equation and understand moles, you can do calculations. You can figure out:
- How much product you can make from a certain amount of starting material.
- How much starting material you need to make a certain amount of product.
- How much of one reactant is needed to completely react with another.
These calculations help scientists predict the outcomes of experiments and industrial processes.
See also
 In Spanish: Estequiometría para niños
In Spanish: Estequiometría para niños
 | Kyle Baker |
 | Joseph Yoakum |
 | Laura Wheeler Waring |
 | Henry Ossawa Tanner |