Supersaturation facts for kids
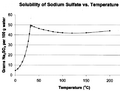
Supersaturation in chemical solutions is when a solution contains more of a solute than can be dissolved in it. This usually makes some of the solute separate itself from the solution.
Examples
- Rain happens in a supersaturated solution of water in air.
- Carbonated water is a supersaturated solution of carbon dioxide gas in water. It is stored at very high pressure in a bottle. This lets the water dissolve more carbon dioxide gas than it can at normal pressure. When the bottle is opened, the pressure falls to the normal atmospheric pressure, and the carbon dioxide gas escapes slowly. Certain things such as small bubbles in the bottle can make this process faster. Such bubbles can be caused by shaking the bottle. Another solute such as sugar powder or a widget can also trigger precipitation.
- A Diet Coke and Mentos eruption is an extreme example. Some drinks such as ales and stouts rely on this effect to produce the 'head' on the surface of the poured product.
Images for kids
See also
 In Spanish: Sobresaturación para niños
In Spanish: Sobresaturación para niños

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Supersaturation Facts for Kids. Kiddle Encyclopedia.