Hawaiian happy-face spider facts for kids
Quick facts for kids Hawaiian happy-face spider |
|
|---|---|
 |
|
| Scientific classification |
The Theridion grallator, also known as the Hawaiian happy-face spider, is a small spider that lives on the Hawaiian Islands. It gets its fun name from the special patterns on its back, which often look like a smiling face. In the Hawaiian language, it's called nananana makakiʻi, which means "face-patterned spider." The second part of its scientific name, grallator, is Latin for "stilt walker". This refers to its long, thin legs.
These spiders are very interesting because they come in many different colors and patterns. Scientists study them to learn more about how living things change over time. What's even cooler is that the spider's color can change for a short time depending on what it eats!
Contents
About the Happy-Face Spider
The happy-face spider is tiny, usually less than 5 millimeters long. That's smaller than your pinky nail! It has long, skinny legs and a body that is usually clear yellow. These long legs make it look quite different from other spiders in its group. This unique look probably helps it survive in its home.
The spider's back is often pale, clear yellow. It can also have red, white, or black patterns on it. Some spiders have patterns that truly look like a smiley face or a grinning clown. Each spider has its own unique pattern, and these patterns can even be different from island to island. Some happy-face spiders have no patterns at all. The color of their back can change from clear yellow to green or orange. This depends on what they have eaten. Having many different colors and patterns helps these spiders hide from birds. Birds often look for a specific color or pattern when hunting. If spiders come in many different looks, it's harder for birds to find them.
Spider Colors
A cool thing about the happy-face spider is its many different colors and patterns on its back. The number of plain spiders compared to patterned ones stays pretty much the same all year. It also stays the same in different places, no matter the weather or how high up they live. This means something in nature helps keep these numbers balanced.
Even though the colors look the same on different Hawaiian islands, the genes that cause them can be different. Scientists put these colors into groups based on the main color or pattern. Some groups are Yellow, Red front, Red back, Red lines, Red ring, Black ring, and White.
How Genes Affect Color
The colors of these spiders are passed down through simple Mendelian genetics, like how you might inherit eye color. The most common color is Yellow, found in about 70% of these spiders. Studies show that the Yellow color, which is also the "unpatterned" look, is a weaker trait compared to all the patterned colors.
For the patterned spiders, the more color they have, the stronger their related gene is. Genes for black, red, or white colors are very strong. The White color is usually the strongest of all. The white color comes from a lot of a substance called guanine under the spider's skin. Guanine is a waste product in spiders. This white layer helps when bright colors are good for hiding. Guanine is only found under the red and black colors that make up the patterns. The White and Red lines colors are equally strong. Male and female spiders have the same chance of having these colors.
The patterns on the spider's main body (back) are controlled by one main gene. Stronger genes often make one pattern appear over another. For example, red and black patterns with guanine deposits help the spider blend in.
Spider Family and History
Close Relatives
Scientists have found at least nine types of spiders in Hawaii that are related to the happy-face spider. They think these spiders came from the Americas. The happy-face spider's closest relatives are other Hawaiian spiders like Theridion posticatum and Theridion kauaiense. The happy-face spider and a spider called Theridion californicum both have one common, plain color (Yellow for the happy-face spider) and other less common, more noticeable colors. Scientists believe the happy-face spider group might be more closely related to a different group of spiders called Exalbidion than to other Theridion spiders. It's thought that the happy-face spider first appeared about 4.22 million years ago.
Most Hawaiian Theridion spiders are closely related. There's also an unnamed Theridion spider that looks a bit like the happy-face spider, with long legs and a similar body shape. This means it might have faced similar challenges as it evolved. These spiders also build their webs in a very similar way. Scientists are still working to properly group these spiders.
Spider Populations on Different Islands
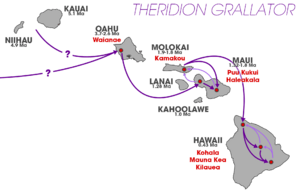
The genes for the happy-face spider's colors are different on different islands, even though the colors themselves look the same. On Maui, one gene controls all the colors. But on Hawai’i Island, at least two different genes are involved. Also, on Hawai’i, some colors show up differently in males and females. For example, a spider might be Yellow if it's a female but Red front if it's a male, even with the same genes. These differences are likely because the genes are used differently in males and females, not because they are linked to sex.
The different genetic backgrounds on Maui and Hawai’i are because the islands formed at different times. Maui is older than Hawai’i. The fact that some colors depend on sex on Hawai’i, but not on Maui, suggests that how these traits are passed down changed over time. Today, spiders don't move much between the Hawaiian islands. This is why each island has its own unique groups of spiders. But if spiders from different islands mate, they can still have healthy babies. This shows that the happy-face spiders on Maui and Hawai’i are not too different from each other yet.
How They Evolved
The reason for the happy-face spider's many colors is a bit of a mystery. But we know that certain pressures from nature affect how many of each color there are. The Yellow spider is very common, making up about 70% of the population. The rest are the patterned spiders. This high number of Yellow spiders suggests it's very important for their survival.
The main idea is that predators, like birds, play a big role. Happy-face spiders live under green leaves. The Yellow color helps them blend in with the sunlight shining through the leaves, making it harder for predators to see them. But the other colors also have benefits, even if they are less common. This can also be explained by how predators hunt.
Female spiders benefit more from being Yellow because they don't move much. They stay on their leaves most of the time. Male spiders, however, move around a lot on the ground looking for mates. When they are not under a leaf, the Yellow color might not always be the best for hiding. Some of the rarer patterned colors might actually help males stand out less to predators. So, if there are too many Yellow females, they might start to prefer these less common, patterned males. This helps keep all the different colors in the population.
The different ages and environments of the Hawaiian islands have helped create these many color variations. Newer islands have less genetic variety, while older islands have much more.
Home and Where They Live
Habitat
Happy-face spiders live in wet and moist forests. Wet places get about 200 to 350 centimeters of rain each year, and moist places get 100 to 200 centimeters. You can find them in the forests of O’ahu, Moloka’i, Maui, and Hawai’i. They like to live under the leaves of plants like the native Broussaisia arguta and Clermontia arborescens. They also like the introduced Hedychium coronarium. The large, slippery leaves of H. coronarium are especially good for helping the spiders hide from predators.
These spiders have also been seen in "kipukas," which are areas of land surrounded by old lava flows. However, they don't live in the fresh lava flows themselves.
Where They Are Found
The happy-face spider is only found in the Hawaiian Islands. Small groups of them have been seen in the rainforests of O’ahu, Moloka’i, Maui, and Hawai’i. They live at heights between 300 and 2000 meters.
The number of different color patterns varies a bit between Maui and Hawai’i. On Maui, the most common patterned spider has a red "U" shape on its front back. On Maui, both male and female spiders can be Yellow, Red front, Red blob, or Red ring. But on Hawai’i, these colors are linked to sex. Yellow and Red blob are only found in females, while Red front and Red ring are only found in males. The same genes control these colors in both sexes, but they show up differently.
What They Eat
Color Change from Food
Happy-face spiders can change color depending on what they eat. This happens because their backs are somewhat clear. Like most spiders, the back of the happy-face spider is thin and see-through. So, you can sometimes see what they've eaten inside their bodies. Usually, digested food looks dark brown or black.
Sometimes, colors from their food get stored in the spider's skin. These colors might be dull or bright. A common change is from clear yellow to orange. This often happens because they eat a lot of flies (about 70% of their diet). If they eat other types of prey, they might temporarily turn dark brown. These food-related colors can stay for two to six days. Once the food is digested and leaves their body, the spider's back returns to its original clear pale yellow.
How Adults Hunt
Happy-face spiders don't use webs to catch food. They don't just sit and wait like many web-building spiders. Instead, they move around freely, often going to nearby leaves to find insects. When they catch prey, they use their silk. They often eat small flies. The type of plant they live on doesn't change what they prefer to eat. However, depending on the plant, their hunting style might change. For example, on Hedychium leaves, these spiders are more aggressive when hunting, even if they don't catch as much prey as on other plants.
Who Eats Them
Carnivorous caterpillars from the Eupithecia group have been seen attacking happy-face spiders. Several types of these caterpillars in Hawaii hunt happy-face spiders. These caterpillars lie on leaves and attack spiders that touch their bodies. When attacked, the happy-face spider tries to bite the caterpillar and run away.
The Eleutherodactylus coqui is an invasive frog from Puerto Rico that also eats happy-face spiders. These frogs were first seen in Hawaii in the 1980s.
Spider Webs
The happy-face spider lives under plant leaves and spins a very small web. This web is flat and usually found on the underside of a leaf or sometimes in tree cracks. Happy-face spider webs are often very flimsy and tangled. This is common for spiders in the Theridiidae family.
Their webs are not used much, possibly because Hawaii's rainy weather damages the sticky silk, making it hard to catch prey. Instead of using the web to find food, the happy-face spider finds prey by sensing vibrations that the prey makes on the leaf. The spiders can then figure out where the prey is.
Even though they use their webs very little, happy-face spiders can still use their silk to catch prey. They sense vibrations, move close to the prey, quickly turn around, and throw silk onto the prey to wrap it up. The silk is sticky, which helps them catch food well. Also, mother happy-face spiders might use webs to protect their egg sacs or store food for their babies.
Reproduction and Life Cycle
When a female happy-face spider is about to have her last molt (shed her skin), a mature male might join her on the same leaf. After she finishes molting, they mate. A few weeks later, the female lays her egg sacs. She stays very close to them with a short silk thread until the eggs hatch. When the eggs are ready, the mother spider loosens the silk around them so the baby spiders can come out.
The number of happy-face spiders changes with the seasons. In winter (October to March), there are more small, young spiders. In spring (May to August), there are more adult spiders, and most of them are mothers with egg sacs. In fact, up to 85% of the spiders in a group can be mothers with egg sacs during these months.
The patterns on young spiders are different from those on adult spiders. Young spiders can have black or maroon patterns that adults don't. The Red blob pattern, where the whole back is red, is much more common in adult happy-face spiders. This means that the maroon and black patterns on young spiders likely turn into the Red blob pattern as they grow up.
Mating
Male and Female Interactions
Adult male spiders actively move through the forest looking for females, who tend to stay in one place. Courtship mostly involves vibrations and smells. For example, males might do a special dance with body movements and by plucking the web. Females judge these vibrations during the dance. Mating happens at night, with both spiders hanging under a leaf. Males usually die soon after mating. Females live longer and guard their eggs until they hatch, catching food for their young.
Sometimes, females prefer rarer male patterns for mating. This might be because a less common pattern helps the male hide from predators better. This rare pattern might then become more common until predators start to notice it. This idea, called apostatic selection, helps keep many different patterns in the spider population. This advantage for rare males might be stronger for males because they move around a lot during mating season.
Happy-face spiders don't see very well. So, their preference for rarer patterns isn't about how good-looking the male is. Instead, it's about the advantage these patterns give against predators. Because they have poor eyesight, males use vibrations and smells to court females.
Parental Care
Egg Guarding
A mother happy-face spider is very protective of her young right after they are born and while she is guarding her egg sac. She needs to protect her babies from predators, parasitic wasps, and even the leaf falling off. Once the baby spiders hatch, the mother continues to defend and care for them. She shows great maternal care by feeding all the baby spiders together and protecting them from danger.
Baby spiders stay on the same leaf with their mother for about 40 to 100 days. During this time, they can't catch their own food and will die if their mother isn't there. The mother wraps all the food she catches in her silk and is never seen eating the food herself.
This strong guarding behavior helps more eggs hatch successfully. If a mother happy-face spider dies or leaves her egg sac, the egg sac is usually eaten by a predator in less than a week. When a mother stays and guards her egg sac, about 57.2% of the eggs hatch. This shows how important egg guarding is.
Adoption
Mothers will accept and care for egg sacs that are not their own. If baby spiders are moved to a new group, the new mothers will "adopt" them and care for them as if they were their own. Adoption might happen if a mother spider is lost, usually because of predators or old age. Baby spiders who lose their mother might try to survive alone or move to other leaves to join another family. Mothers are very welcoming to adopted baby spiders, no matter their color pattern. Also, there isn't much fighting for food within a group, which makes it easier for adopted spiders to be accepted.
Parent-Offspring Challenges
Sometimes, there can be a conflict between a mother and her babies over how much care she gives. If a mother happy-face spider has a second group of babies, she has to stay with the first group for a while after they hatch because they can't feed themselves. This means the second group of babies might not get as much care because the mother is still busy with the first group.
Social Behavior
Adult female spiders usually stay in one place, under leaves, while males move around more to find mates. Because males move more, they are often more noticeable to predators. Pregnant females and females guarding egg sacs will never share a leaf with other adult happy-face spiders.
Spiders from the same group have not been seen fighting over food. Also, baby spiders killing each other or eating each other has not been observed.
Parasites
Happy-face spiders often get attacked by tiny wasps from the Baeus group. These wasps also attack other spiders. The wasps lay their eggs inside the spider's eggs, causing many spider eggs to die. Because the wasp eggs are so small, mothers might have trouble knowing if their egg sacs have been attacked. Baeus wasps can still lay their eggs even when the mother spider is guarding her eggs.
|