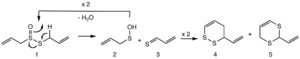Vinyldithiin facts for kids
Vinyldithiins are special natural chemicals found in garlic. They are made when garlic is crushed and its powerful compounds break down. These chemicals are part of what gives garlic its unique smell and taste.
Vinyldithiins are also found in some garlic oil supplements. These supplements are made by soaking crushed garlic in oil. This process helps create and keep the vinyldithiins.
What Happens When You Crush Garlic?
When you crush a garlic clove, a special helper called an enzyme is released. This enzyme is called alliinase. It helps turn another compound in garlic, called alliin, into a new chemical called allicin.
Allicin is very important because it's what makes garlic so strong and healthy. But allicin doesn't stay the same for long. It quickly breaks down into many other interesting chemicals.
How Vinyldithiins Are Made
When allicin breaks down, especially if there's oil around, it forms several new compounds. Two of these are the vinyldithiins. Another important one is called ajoene.
Here's a simple way to think about it:
- First, allicin breaks apart into two smaller pieces.
- One of these pieces is called thioacrolein.
- Then, two of these thioacrolein pieces join together. They connect in a special way to form the vinyldithiins. This joining process is a type of chemical reaction.
Garlic cloves have a good amount of allicin. A single crushed garlic clove can have several milligrams of allicin. This means there's plenty to break down and form vinyldithiins!
What Happens When Vinyldithiins Get Hot?
If vinyldithiins are heated to very high temperatures (over 400 degrees Celsius), they can break apart again. When they break, they turn back into the smaller thioacrolein pieces that formed them. This is like undoing the joining process.