Adiabatic process facts for kids
An adiabatic process is a special kind of change in thermodynamics. It happens when a fluid (like air or water vapor) gets warmer or cooler without actually taking in or giving out heat from its surroundings. Instead, its temperature changes because its pressure or volume changes.
A common example is how clouds form. When warm, moist air rises, it expands because the air pressure is lower higher up. As it expands, it cools down. This is called adiabatic cooling. If it cools enough, the water vapor in the air turns into tiny water droplets or ice crystals, forming clouds. The opposite happens when cold air sinks. As it sinks, it gets compressed by the higher air pressure closer to the ground. This makes it warm up, which is called adiabatic warming. This warming can make clouds disappear or cause rain to evaporate before it reaches the ground.
What is an Adiabatic Process?
Imagine you have a system, like a sealed container with air inside, that is perfectly insulated. This means no heat can get in or out. If you quickly expand or squeeze the air inside this system, the air's temperature will change. This change happens because the air is doing work or work is being done on it, not because heat is moving in or out.
In real life, nothing is perfectly insulated. But sometimes, heat moves so slowly that we can pretend it's an adiabatic process. This is especially true for very fast changes, like air moving quickly in the atmosphere.
This idea is based on the first law of thermodynamics. This law tells us how energy is conserved. It says that the change in a system's internal energy (how much energy is stored inside) is equal to the heat added to the system minus the work done by the system.
In an adiabatic process, no heat is added or removed. So, the change in internal energy is directly related to the work done. If the system does work (like expanding), its internal energy goes down, and it cools. If work is done on the system (like compressing it), its internal energy goes up, and it warms.
Think of it like this:
- When air expands, it pushes outwards, doing work. This uses up some of its internal energy, so it cools down.
- When air is compressed, something pushes on it, doing work on the air. This adds energy to the air, so it heats up.
Adiabatic Process for Ideal Gases
Scientists often study gases using a simplified model called an "ideal gas." This helps us understand how gases behave in different situations, including adiabatic processes.
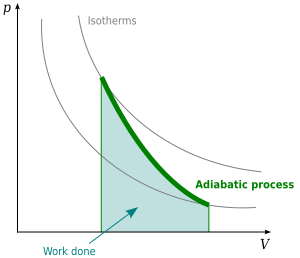
For an ideal gas undergoing an adiabatic process, there's a special relationship between its pressure and its volume. This relationship is often written as: 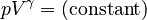
Here:
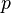 is the pressure of the gas.
is the pressure of the gas.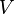 is the volume of the gas.
is the volume of the gas.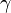 (gamma) is a special number that depends on the type of gas. It's the ratio of the gas's heat capacity at constant pressure to its heat capacity at constant volume.
(gamma) is a special number that depends on the type of gas. It's the ratio of the gas's heat capacity at constant pressure to its heat capacity at constant volume.
This equation means that if the volume of an ideal gas changes during an adiabatic process, its pressure will also change in a very specific way. For example, if the volume increases, the pressure will decrease, and vice versa. This relationship helps scientists predict how gases will behave in many real-world situations, from engines to weather patterns.
See also
 In Spanish: Proceso adiabático para niños
In Spanish: Proceso adiabático para niños