Beer–Lambert law facts for kids
The Beer-Lambert law helps scientists measure how much of a specific substance is in a liquid or gas. It's often called Beer's law or the Bouguer–Lambert law. This law explains how light gets weaker as it travels through a material. It's used in many areas, from chemical analysis to understanding how light travels through space.
Contents
History
The idea behind this law started a long time ago with two different scientists.
Bouguer's Observations
Before 1729, a French scientist named Pierre Bouguer studied how light loses its brightness as it passes through different materials. He noticed that the more material the light went through, the weaker it became. He also saw that if the light was already weak, it would lose less brightness than if it was very bright to begin with.
Later, in 1760, a German scientist named Johann Heinrich Lambert wrote about Bouguer's work. Lambert put Bouguer's observations into a mathematical form that we still use today. He showed that the amount of light lost is directly related to how bright the light is and how far it travels through the material.
Beer's Observations
Much later, in 1852, another German scientist named August Beer studied how light is absorbed by colored liquids. He wanted to know how much light a specific colored substance would absorb.
Beer found that if you had a strong solution, you could figure out how much light it absorbed by measuring a weaker solution. He realized that the light weakened in a specific way, like a pattern. If the light lost half its brightness over a certain distance, it would lose half of the *remaining* brightness over the next same distance. This is what we call an "exponential relationship."
Combining the Ideas
The modern Beer-Lambert law combines the ideas from both Bouguer and Beer. It connects how much light is absorbed (called "absorbance") to two main things:
- The amount of the substance that absorbs light (its concentration).
- The distance the light travels through the substance (the "path length").
This combined law helps scientists understand and measure how different materials interact with light.
How it Works
Imagine you shine a flashlight through a colored glass. The light that comes out on the other side will be weaker than the light you shined in. The Beer-Lambert law helps us understand *why* and *how much* weaker it gets.
The amount of light that gets weaker depends on:
- How many particles are in the material that can block or absorb the light.
- How much each particle blocks or absorbs the light.
For example, if you have a very dark colored drink, it has many particles that absorb light, so less light will pass through. If you have a lighter drink, fewer particles absorb light, and more light will pass through.
Solutions vs. Other Materials
- Beer mainly studied liquids, like colored water. In these liquids, light mostly gets weaker because it's *absorbed* by the colored particles. The light doesn't usually scatter much.
- Bouguer looked at things like light from stars passing through the atmosphere. In this case, light gets weaker not just from being absorbed, but also from being *scattered* by tiny particles in the air. When light scatters, it bounces off in different directions and doesn't reach the detector.
When light is both absorbed and scattered, we call the total weakening "attenuation."
The Main Formula
The most common way to write the Beer-Lambert law is: 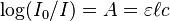 Let's break down what these letters mean:
Let's break down what these letters mean:
- A is the absorbance. This tells you how much light the substance absorbed.
- ε (epsilon) is the molar absorptivity (or "extinction coefficient"). This is a special number that tells you how strongly a specific substance absorbs light at a certain wavelength. Every substance has its own epsilon value.
- ℓ (ell) is the path length. This is the distance the light travels through the substance, usually measured in centimeters.
- c is the concentration. This is how much of the substance is dissolved in the liquid, usually measured in moles per liter.
So, if you know how strongly a substance absorbs light (ε), and you measure how much light it absorbs (A) when it travels a certain distance (ℓ), you can figure out its concentration (c)! This is super useful in chemistry labs.
When the Law Might Not Work Perfectly
The Beer-Lambert law is a great tool, but it doesn't always work perfectly. Here are some reasons why:
- Too much substance: If the liquid is too concentrated, the particles might be too close together and start affecting each other. This can make the law less accurate.
- Scattering: If the material scatters a lot of light (like a cloudy liquid), the law might not work well because it assumes light is only absorbed, not scattered.
- Special light: The law works best if the light used is very specific (like a single color or wavelength) and not too strong. If the light is too intense, it can change the molecules it's trying to measure.
For the law to work best, the light should travel in straight, parallel lines, and the substance should be evenly mixed.
How it's Used in the Atmosphere
The Bouguer-Lambert law is also used to understand how sunlight or starlight gets weaker as it travels through Earth's atmosphere. When light comes from the sun or stars, it passes through different gases and tiny particles in the air.
The weakening of light in the atmosphere happens because of:
- Aerosols: Tiny particles like dust or smoke that can absorb and scatter light.
- Gases: Gases like carbon dioxide and ozone that absorb specific colors of light.
- Scattering: Light scattering off air molecules (like nitrogen and oxygen) is why the sky looks blue!
Scientists use this law to figure out how much of these different things are in the atmosphere. This helps them understand air pollution and how aerosols affect our climate.
See also
 In Spanish: Ley de Beer-Lambert para niños
In Spanish: Ley de Beer-Lambert para niños
- Applied spectroscopy
- Atomic absorption spectroscopy
- Absorption spectroscopy
- Cavity ring-down spectroscopy
- Clausius-Mossotti relation
- Infra-red spectroscopy
- Job plot
- Laser absorption spectrometry
- Lorentz-Lorenz relation
- Logarithm
- Polymer degradation
- Scientific laws named after people
- Quantification of nucleic acids
- Tunable diode laser absorption spectroscopy
- Transmittance#Beer–Lambert law
 | William Lucy |
 | Charles Hayes |
 | Cleveland Robinson |

