Charcot–Marie–Tooth disease facts for kids
Quick facts for kids Charcot–Marie–Tooth disease |
|
|---|---|
| Synonyms | Charcot–Marie–Tooth neuropathy, peroneal muscular atrophy, Dejerine-Sottas syndrome |
 |
|
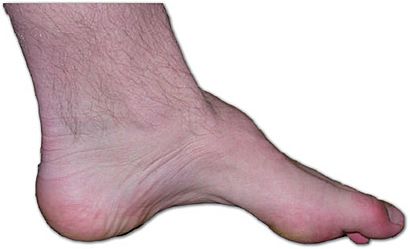
| The foot of a person with Charcot–Marie–Tooth disease: The lack of muscle, a high arch, and claw toes are signs of this genetic disease. | |
| Pronunciation |
|
| Symptoms | Foot drop, hammertoe, peripheral muscle wasting of lower legs and lower arm/hands |
| Usual onset | Childhood – early adulthood |
| Duration | Lifelong |
| Causes | Family history (genetics) |
| Risk factors | Family history (genetics), high-arched feet, flat-arched feet |
| Diagnostic method | Genetic testing, nerve conduction study or electromyogram (EMG) |
| Similar conditions | Muscular dystrophy |
| Treatment | Management to maintain function |
| Prognosis | Progressive |
| Frequency | Prevalence: 1 in 2,500 |
Charcot–Marie–Tooth disease (CMT) is a group of inherited disorders that affect your nerves. It gradually causes muscles to weaken and makes it harder to feel things, especially in the arms and legs. CMT is the most common inherited nerve disorder. It affects about 1 in 2,500 people.
The disease is named after the doctors who first described it: Jean-Martin Charcot, Pierre Marie, and Howard Henry Tooth. There is no cure for CMT. However, treatments help people manage their symptoms and stay active.
Contents
What are the Signs and Symptoms of CMT?
Symptoms of CMT usually start when a person is a child or young adult. For some, symptoms might not appear until their 30s or 40s.
- Often, the first sign is a foot drop. This means it's hard to lift the front part of the foot.
- People might also have high arches in their feet or hammertoe, where toes are always curled.
- Muscles in the lower legs can become weak and shrink. This can make legs look very thin, like a "stork leg."
- As the disease progresses, hands and forearms can also become weak.
- It can be harder to feel touch, pain, or temperature in the feet, ankles, legs, hands, and arms.
- Some people experience painful muscle cramps.
- High-arched feet (pes cavus) or flat feet (pes planus) are common with CMT.
- Overusing an affected hand or limb can make symptoms worse. This can include numbness, muscle spasms, and painful cramping.
The symptoms and how fast the disease progresses can be different for everyone.
- Some people might grind their teeth or squint without realizing it.
- In some cases, CMT can affect breathing, hearing, vision, and muscles in the neck and shoulders.
- A curved spine (scoliosis) is also common.
- Hip joints can sometimes be shaped differently.
- Some people might have stomach or digestion problems.
- It can also make chewing, swallowing, or speaking difficult.
- Muscles might shake (tremor) as they get weaker.
- Long periods of not moving, like after an injury, can make CMT symptoms worse.
Pain is common for people with CMT. It can come from changes in posture, bone problems, tired muscles, and cramps. Physical therapy, surgery, and special devices can help. Sometimes, pain medicine is also needed.
What Causes Charcot–Marie–Tooth Disease?
CMT is caused by changes (called mutations) in certain genes. These genes are important for making proteins that help nerves work correctly.
Nerve signals travel along a part of the nerve cell called an axon. The axon is covered by a protective layer called a myelin sheath. Most gene changes in CMT affect the myelin sheath. Some changes, however, affect the axon itself.
How is CMT Classified?
CMT is a complex disease. The gene changes linked to it can happen in many different genes. Because of this, CMT is grouped into several types and subtypes based on which gene is affected.
Chromosome 17 and Nerve Problems
The most common cause of CMT (70–80% of cases) is an extra copy of a section on chromosome 17. This section includes a gene called PMP22.
Some gene changes affect the MFN2 gene on chromosome 1. This gene makes a protein for mitochondria, which are like the power plants of cells. When MFN2 is changed, mitochondria can clump together. This stops them from traveling down the axon to the synapses (where nerve signals are passed). This means the synapses can't work properly.
X-linked CMT and Schwann Cells
CMT can also be caused by gene changes on the X-chromosome. This is called X-linked CMT (CMTX). In CMTX, changed proteins called connexons create faulty connections between cells. This stops important molecules and signals from moving between cells.
One common change is in the GJB1 gene. This gene makes a protein called connexin 32. This protein is found in Schwann cells. Schwann cells are special cells that create the myelin sheath by wrapping around the axon. When the myelin sheath is damaged, nerve signals travel slower. Doctors can measure this with a test called electromyography.
GARS1-Related Axonal Neuropathy Some types of CMT, like CMT2, involve damage to the nerve axons themselves, not just the myelin sheath. Damaged axons slow down signals to muscles and the brain. This can cause muscle weakness, loss of feeling, and foot problems. Symptoms of CMT2 usually appear between ages 5 and 25.
CMT2D is one type of CMT2. It is diagnosed when a person has both muscle weakness and loss of sensation. Symptoms can include foot problems, muscle weakness and cramps, weaker reflexes, and muscle shrinking. The symptoms and how severe they are can vary a lot from person to person.
CMT2D is caused by changes in the GARS1 gene. This gene makes a protein called glycyl-tRNA synthetase (GlyRS). This protein is important for making other proteins in the body. When the GARS1 gene is changed, it can stop proteins from being made correctly. This leads to the nerve problems seen in CMT2D.
GARS1-related nerve problems get worse over time. Scientists are still learning how these gene changes cause the disease.
How is CMT Diagnosed?
Doctors can diagnose CMT using a few different tests:
- Nerve conduction studies: These tests measure how fast nerve signals travel.
- Nerve biopsy: A small piece of nerve tissue is removed and looked at under a microscope.
- DNA testing: This is a blood test that looks for specific gene changes. DNA testing can give a clear diagnosis. However, not all the gene changes for CMT are known yet.
CMT is often first suspected when someone develops weak lower legs or foot problems. These include foot drop, hammertoes, or high arches. If these signs appear, a person should see a doctor who specializes in neurology or rehabilitation.
To check for muscle weakness, the doctor might ask you to walk on your heels or push your leg against their hand. To check for loss of feeling, they might test your reflexes, like the knee jerk. Reflexes are often weaker or missing in people with CMT. The doctor will also ask about your family's health history, as CMT is often passed down in families.
How is CMT Managed?
The main goal for people with CMT is to keep their movement, muscle strength, and flexibility. A team of healthcare professionals often works together to help. This team might include:
- Occupational therapists (OT): They help with daily activities and saving energy.
- Physical therapists (PT): They focus on strengthening muscles, stretching, and exercise.
- Orthotists: They make special braces or devices.
- Podiatrists: They care for feet.
- Orthopedic surgeons: They perform surgeries if needed.
Physical therapy helps create an exercise plan that fits each person's needs.
Orthotics
If the leg muscles are weak, special braces called orthotics can be very helpful. These are often custom-made. For example, if the muscles that lift the foot are weak, an ankle-foot orthosis (AFO) can help control foot drop. These braces can improve balance and make walking easier.
Proper footwear is also very important for people with CMT. However, it can be hard to find shoes that fit well because of high arches and hammertoes. Because people with CMT might have less feeling in their feet, they may need to see a podiatrist for help with nail care or calluses.
Sometimes, surgery can help stabilize feet or correct problems that get worse over time. These surgeries might involve straightening toes, lowering the arch, or fusing the ankle joint for stability.
People with CMT need to be extra careful to avoid falls. Fractures can take longer to heal. Also, being inactive for long periods can make CMT symptoms worse. It is important for CMT patients to avoid the chemotherapy drug vincristine, as it can be harmful.
What is the Prognosis for CMT?
The severity of CMT symptoms can vary a lot, even among people with the same type of CMT. Some people might have very mild or no symptoms at all. For most cases, CMT is not known to change how long a person lives.
History of CMT
The disease is named after the three doctors who first described it in the late 1800s:
- Jean-Martin Charcot (a Frenchman)
- Pierre Marie (his student)
- Howard Henry Tooth (a Briton)
See also
 In Spanish: Síndrome de Charcot-Marie-Tooth para niños
In Spanish: Síndrome de Charcot-Marie-Tooth para niños
- Charcot–Marie–Tooth disease classifications
- Hereditary motor and sensory neuropathies
Images for kids
-
Denervation atrophy of type II muscle fibers