Clinical trial facts for kids

Clinical trials are special studies that test new treatments, medicines, or ways to help people stay healthy. These studies involve people and help scientists find out if new things like vaccines, medicines, special diets, or medical devices (like a new type of hearing aid) are safe and work well. They also compare new treatments with old ones.
Clinical trials collect information to see how safe and helpful these new ideas are. Before a trial can start, special groups of experts (like health authorities or ethics committees) must approve it. These groups make sure the trial is fair and safe for the people taking part. Their approval means the trial can happen, but it doesn't mean the new treatment is already proven to be safe or effective.
Most clinical trials test new products or ways to help people. Sometimes, a country's health group needs to see the results of a clinical trial before they allow a new medicine or treatment to be sold to everyone.
Contents
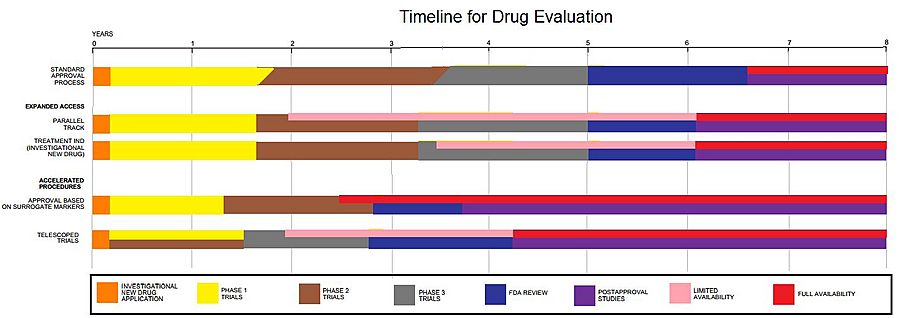
How Long Do Trials Last?
Clinical trials are just one part of creating a new treatment. For example, new medicines are first found, cleaned, studied, and tested in labs. This happens long before they are ever tested in people.
It's a long process! About 1,000 possible medicines are tested in labs before just one is ready for human clinical trials. For example, a new cancer medicine usually has about six years of research behind it before it even gets to the clinical trial stage.
Trials can involve a few volunteers at first, then grow to include many more people. They can be small or very large, taking place in one hospital or many hospitals around the world. Scientists design these studies carefully to make sure the results are correct and can be trusted.
Clinical trials can be very expensive. A government group or a company that makes medicines or medical devices usually pays for them. Only about 10 out of every 100 medicines that start human clinical trials actually get approved for use.
Who Takes Part in Trials?
Some clinical trials involve healthy people who don't have any health problems. Other trials are for patients who have specific health conditions and want to try a new treatment.
Healthy volunteers in early trials usually get a small payment. This payment is for their time and effort, not for any risk. They might have to stay at a study center for a few nights.
In most other trials, people are not paid to take part. This is to make sure they are joining because they hope to get better or help medical science, not just for money. However, they might get small payments for things like travel costs or for their time when they give updates on their health.
Everyone who wants to join a trial must have a health check-up. The checks depend on the trial, but they often include:
- Checking the heart's electrical activity (ECG)
- Measuring blood pressure, heart rate, and body temperature
- Taking blood samples
- Taking urine samples
- Measuring weight and height
All volunteers have the right to know and understand everything about the trial before they agree to join. This is called informed consent.
Clinical trials can be designed to:
- See if a certain medicine helps treat a disease.
- Find out if a disease or treatment gets better with a different amount of medicine.
- Check if a medicine already used for one disease can also help treat another disease.
- Compare a new medicine or treatment with the usual treatment to see which is better.
- Compare two medicines or treatments to see which one works best for a condition.
History of Clinical Trials
The idea of clinical trials started a very long time ago. A scientist named Avicenna wrote about rules for testing medicines in 1025 AD. He created a clear guide for how to experiment and prove if medicines worked. His rules are still important for modern clinical trials today:
- The medicine must be pure and not have anything extra in it.
- It should be used for a simple disease, not a mix of problems.
- The medicine should be tested on two opposite types of diseases.
- The strength of the medicine must match the strength of the disease.
- Scientists must watch how long it takes for the medicine to work.
- The medicine's effect must happen often, not just by chance.
- The tests must be done on humans, because what works for animals might not work for people.
One very famous clinical trial happened in 1747. A doctor named James Lind showed that oranges and lemons could cure a disease called scurvy. He gave different things like vinegar or cider to groups of sick sailors. He found that the sailors who ate oranges and lemons got much better in just six days.
Images for kids
-
Edward Jenner giving a vaccine in 1796.
See also
 In Spanish: Ensayo clínico para niños
In Spanish: Ensayo clínico para niños