Diastereomer facts for kids
| Diastereomers | |
|---|---|
 |
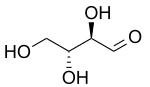 |
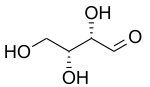
| D-Threose | D-Erythrose |
Diastereomers (pronounced dye-uh-STER-ee-oh-mers) are special kinds of isomers. Isomers are molecules that have the same chemical formula but different arrangements of their atoms. Diastereomers are stereoisomers, meaning their atoms are connected in the same order, but they are arranged differently in 3D space.
The key thing about diastereomers is that they are not enantiomers. Enantiomers are like mirror images of each other, similar to your left and right hands. Diastereomers, however, are not mirror images. They have different 3D shapes because of how their atoms are arranged around certain points called chiral centres.
Contents
What Makes Diastereomers Different?
Molecules often have special spots called chiral centers. Think of a chiral center as a carbon atom connected to four different groups. Because these groups are all different, they can be arranged in two unique ways, like a left-handed or right-handed version.
- If a molecule has only one chiral center, it will have two forms that are mirror images of each other. These are enantiomers.
- If a molecule has two or more chiral centers, things get more interesting.
- When all the chiral centers in a molecule are flipped to their opposite arrangement, you get an enantiomer.
- When only some of the chiral centers are flipped, but not all of them, you get a diastereomer.
This means diastereomers have the same atoms and bonds, but their 3D shapes are different in at least one chiral center, but not all of them.
Diastereomers vs. Enantiomers
It's important to know the difference between diastereomers and enantiomers:
- Enantiomers are mirror images of each other. They have almost identical properties, like boiling points, melting points, and how they react with other non-chiral molecules. The only time they act differently is in a special environment, like inside the human body, or when they interact with light.
- Diastereomers are not mirror images. Because their 3D shapes are more different, they can have very different physical and chemical properties. For example, they might have different melting points, boiling points, or even different solubilities in water. This makes them easier to separate from each other than enantiomers.
Epimers: A Special Type of Diastereomer
Sometimes, diastereomers are different at only one of their chiral centers. These specific types of diastereomers are called epimers.
For example, look at the picture of D-Threose and D-Erythrose at the top of this page. Both of these molecules have two chiral centers.
- D-Threose and D-Erythrose are diastereomers because they are not mirror images of each other.
- They are also epimers because they only differ in the arrangement of atoms around one of their two chiral centers.
Understanding diastereomers helps scientists create new medicines and materials, as the exact 3D shape of a molecule can greatly affect how it works.
See also
 In Spanish: Diastereoisómero para niños
In Spanish: Diastereoisómero para niños

