Enantiomer facts for kids
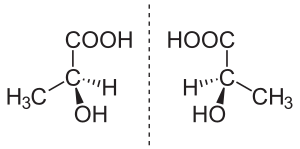
In chemistry, an enantiomer (say: ee-NAN-tee-oh-mer) is a special type of isomer. Think of your left and right hands. They look like mirror images of each other, right? But you can't perfectly stack one on top of the other so they match up exactly. That's a bit like how enantiomers work!
An enantiomer is a molecule that is a mirror image of another molecule. But just like your hands, you can't make them perfectly fit on top of each other. This special property of molecules is called chirality (say: ky-RAL-uh-tee). A molecule is chiral if it has a mirror image that cannot be placed perfectly on top of it.
These mirror-image molecules often have a special spot called a "chiral center." This is usually a carbon atom connected to four different groups of atoms. If a molecule has only one chiral center, then its mirror image is an enantiomer. If it has more than one, things get a bit more complicated, and they might be called diastereoisomers instead.
Contents
What Makes Enantiomers Special?
Even though enantiomers look like mirror images, they can act very differently, especially in living things.
Physical Properties
Enantiomers usually have the same basic physical properties. For example, they will have the same boiling point and melting point. They also react the same way with many common chemicals. This makes them hard to tell apart and separate in a lab.
How They Interact with Light
One interesting way enantiomers are different is how they interact with light. When a special kind of light, called "plane-polarized light," passes through a solution of an enantiomer, the light will twist. One enantiomer will twist the light in one direction (say, clockwise), and its mirror image will twist the light in the exact opposite direction (counter-clockwise). This is one way scientists can identify and measure them.
Biological Effects
The biggest difference between enantiomers often shows up in biology. Our bodies, and many living systems, are chiral themselves. They are like a "right-handed glove." This means they might only fit or react properly with one specific "hand" of a molecule.
- Medicines: In drugs, one enantiomer might be helpful and treat a sickness, while its mirror image might do nothing, or even be harmful. For example, a medicine might have two enantiomers. One helps you feel better, but the other could cause unwanted side effects. Because of this, scientists work hard to separate enantiomers when making medicines.
- Smell and Taste: Enantiomers can also smell or taste different! For example, one enantiomer of a molecule might smell like oranges, while its mirror image smells like lemons. This happens because the smell receptors in our nose are also chiral and react differently to each of the mirror images.
Examples of Enantiomers
Many important molecules in nature exist as enantiomers.
- Amino Acids: The building blocks of proteins in our bodies, called amino acids, are mostly "left-handed" enantiomers.
- Sugars: Most sugars, like glucose, are "right-handed" enantiomers.
- Lactic Acid: This is the acid that builds up in your muscles when you exercise. It also has two enantiomers.
Understanding enantiomers is very important in fields like medicine, food science, and agriculture. It helps scientists create safer and more effective products.
Images for kids
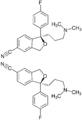
-
Enantiomers of citalopram. The top is (R)-citalopram and the bottom is (S)-citalopram.
See also
 In Spanish: Enantiómero para niños
In Spanish: Enantiómero para niños
 | Selma Burke |
 | Pauline Powell Burns |
 | Frederick J. Brown |
 | Robert Blackburn |