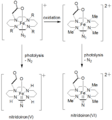High-valent iron facts for kids
High-valent iron refers to special forms of the metal iron that have a very high "oxidation state." Think of the oxidation state as how many electrons an iron atom has given away or taken when it joins with other atoms. For high-valent iron, this number is usually more than 3.
These unique iron compounds also have many bonds – often more than six! A chemical bond is like a tiny glue that holds atoms together. Even with all these bonds, the iron atom usually connects directly to six or fewer other atoms. This is called its "coordination number." It's important to know that high-valent iron is not considered "hypervalent," which is a different chemical idea.
Ferrate was the very first high-valent iron ion (a charged atom or group of atoms) that scientists were able to create.
Contents
What is High-Valent Iron?
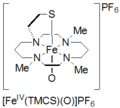
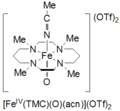
High-valent iron is a fascinating type of iron compound. Normally, iron is found with an oxidation state of +2 or +3. But in high-valent iron, this number can be much higher, like +4, +5, or even +6! This makes these iron compounds very reactive.
Why is it Special?
These special forms of iron are very powerful. They can easily take electrons from other substances. This ability makes them strong "oxidizing agents." Imagine them as tiny electron magnets, pulling electrons away from other chemicals. This process is called oxidation.
How is it Used?
Scientists are very interested in high-valent iron because of its strong oxidizing power. They are studying how these compounds can be used in many ways, such as:
- Cleaning water: They might help break down harmful pollutants in water.
- Making new chemicals: They could be used to create new and useful substances in chemical reactions.
- Understanding biology: High-valent iron forms are also found in some important biological processes inside living things. For example, some enzymes in our bodies use high-valent iron to do their jobs.
Ferrate: The First Discovery
The first high-valent iron compound ever made was called ferrate. Ferrate is an ion where iron has a very high oxidation state, usually +6. Scientists first created ferrate in a lab. This discovery opened up a whole new area of study for chemists. It showed that iron could exist in these unusual and powerful forms.
Different Types of High-Valent Iron
Since the discovery of ferrate, scientists have found many other types of high-valent iron compounds. These often involve iron atoms bonded to oxygen or nitrogen atoms. Each type has slightly different properties and can be used for different purposes. Researchers are always looking for new ways to make and use these interesting iron compounds.
Images for kids