Interpretations of quantum mechanics facts for kids
In the world of quantum mechanics, understanding what the math means in the real world can be tricky. Scientists have many different ideas about what quantum mechanics truly tells us. There's no single way to prove one idea is better than another. But some ideas are more widely accepted by scientists.
Contents
What is Quantum Mechanics?
Main article: Quantum mechanics
Quantum mechanics is a branch of physics that studies the smallest things in the universe. Think of tiny particles like electrons. Two big ideas in quantum mechanics come from scientists named Schrödinger and Heisenberg.
Schrödinger's Wave Equation
One main idea is the Schrödinger equation. This is a special math equation that helps describe tiny particles. It tells us about something called a "wave function." This wave function helps us understand how a particle behaves.
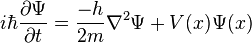
What this equation really means is that a tiny particle, like an electron, isn't just a tiny dot. It also acts like a wave! Imagine a tiny ripple in water. This idea is called wave-particle duality. It's one of the strangest parts of quantum mechanics.
Heisenberg's Uncertainty Principle
Another key idea is the Heisenberg uncertainty principle. This principle is quite strange! It says that you can't know everything about a tiny particle at the same time.
For example, you can't know both the exact position (where it is) and the exact momentum (how fast it's moving and in what direction) of a particle at the same time. If you know its position very well, you'll know less about its momentum. And if you know its momentum very well, you'll know less about its position.
The math for this idea looks like this:
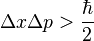
This principle also applies to other things, like energy and time. You can't know the exact energy of a particle at an exact moment in time.
Understanding Probability Waves
One of the most important and weird facts about quantum mechanics is how tiny particles behave. For a long time, scientists didn't know what the "wave function" (from Schrödinger's equation) actually meant.
Then, a physicist named Max Born came up with a brilliant idea. He said the wave function is a probability wave. This means that the wave doesn't tell you exactly where a particle is. Instead, it tells you where the particle is most likely to be found.
Imagine a cloud. Where the cloud is thickest, that's where you're most likely to find the particle. But it could still be found in a thinner part of the cloud.
To find the chance (or probability) of finding a particle in a certain area, scientists use this math:
![\int_a^b \! |\Psi (x, t)|^2 dx=P_{[a,b]}](/images/math/8/e/f/8efb070492c5d6852da876138c29e775.png)
For example, if the chance (![P_{[a,b]}](/images/math/3/1/0/310b6323d7ea094ed3f4656d4ff02b6f.png) ) is 0.5, it means there's a 50% chance of finding the particle in that area. This shows that we can only talk about the probability of finding a particle. We can never say for sure that it will be at one exact spot.
) is 0.5, it means there's a 50% chance of finding the particle in that area. This shows that we can only talk about the probability of finding a particle. We can never say for sure that it will be at one exact spot.
See also
 In Spanish: Interpretaciones de la mecánica cuántica para niños
In Spanish: Interpretaciones de la mecánica cuántica para niños

