Isolated system facts for kids
Imagine a special kind of container that is completely sealed off from everything else around it. In science, this is called an isolated system. It's like a super-duper sealed box where nothing can get in or out – not even tiny bits of matter or any form of energy, like heat or light.
Think of it this way:
- It's a physical system that's so far away from other things that it doesn't interact with them at all.
- It's a thermodynamic system (a part of the universe we are studying) that has really strong, unmoving walls. Through these walls, no mass (stuff) and no energy can pass.
Even though things inside an isolated system might pull on each other with gravity, we usually imagine that no outside forces, like gravity from other planets, can affect it.
Contents
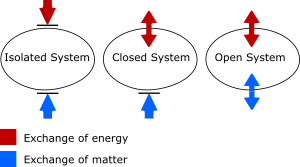
Different Types of Systems
It's helpful to compare an isolated system with other types:
- A closed system is like a sealed thermos. Energy can go in or out (like heat warming up the outside of the thermos), but no matter can escape or enter.
- An open system is like a pot of boiling water without a lid. Both matter (steam escaping) and energy (heat leaving the pot) can move in and out freely.
So, an isolated system is the most "closed off" of all!
Why Are Isolated Systems Important?
In the real world, it's almost impossible to find a truly perfect isolated system. There's always some tiny bit of energy or matter that might sneak in or out, and gravity is everywhere! Because of this, isolated systems are mostly ideal ideas or models that scientists use.
Even though they are just ideas, they are super useful! They help scientists create mathematical models to understand how things work in nature. For example, the planets in our Solar System are often treated as an isolated system when we study their movements, even though they are slightly affected by things outside our solar system. A hydrogen atom (a proton and an electron) can also be thought of as an isolated system, even though it can sometimes interact with light.
Energy and Mass Stay the Same
A cool thing about an isolated system is that its total amount of energy and mass always stays the same. This is called a conservation law. In many cases, scientists treat mass and energy as staying constant separately within these systems.
Scientists also use the idea of isolated systems to understand how things reach a balanced state, called "thermodynamic equilibrium." This is when everything inside the system is stable and not changing.
What About Radiative Isolation?
Sometimes, scientists talk about "radiative isolation." This is about keeping light or other forms of radiation completely inside or outside a space. Imagine a box with walls that are perfect mirrors, reflecting all the light inside so none can escape.
A famous scientist named Max Planck thought about this when studying how heat and light behave in a perfectly sealed box. He imagined walls that were so good at reflecting that they would perfectly isolate the inside from any outside light or energy.
In very extreme conditions, like those found in space, if a perfectly reflective box had enough energy inside, that energy could even create tiny particles of matter! This shows how energy and matter are connected, even in a perfectly isolated space.
See also
 In Spanish: Sistema aislado para niños
In Spanish: Sistema aislado para niños
 | Aaron Henry |
 | T. R. M. Howard |
 | Jesse Jackson |