NMR spectroscopy facts for kids
NMR (Nuclear Magnetic Resonance) spectroscopy is a cool science tool that helps chemists figure out what molecules look like. It's like having a special magnifying glass for tiny atoms! Some atoms, like carbon, hydrogen, and fluorine, act a bit like tiny magnets when they are in a very strong magnetic field. NMR machines can "see" these atoms.
How NMR Works
To use an NMR machine, a chemist puts a tiny bit of a sample into a special glass tube called an NMR tube. They mix it with a special liquid, like heavy water or chloroform-d. Then, this tube goes into the NMR machine.
The machine is a giant magnet, a bit like the MRI machines doctors use to look inside your body. When the sample is inside this strong magnet, some atoms get "excited." When the sample is taken out, these excited atoms calm down and go back to their normal state. The NMR machine has a computer that can detect this change.
This change creates different peaks and bumps on a special diagram. A chemist can print this diagram and study these peaks. Each peak tells them something important about the atoms in their sample. Most NMR machines are designed to look for only one type of atom at a time. For example, an 1H NMR machine only looks for hydrogen atoms, and a 13C NMR machine only looks for carbon atoms.
Understanding an NMR Diagram
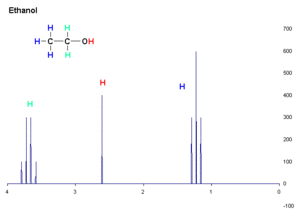
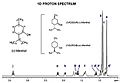
An NMR diagram has a line across the bottom (called the x-axis) that shows something called "shift" in units of "parts per million." Peaks that are further to the left on the diagram are called "downfield." These peaks usually mean that the hydrogen atoms are close to other atoms that pull electrons strongly, like oxygen or nitrogen. They can also be part of a special ring-shaped molecule called an aromatic ring.
Peaks can also split into smaller peaks! This happens if a hydrogen atom has other hydrogen atoms "next door" to it. If there are 'n' hydrogen atoms next to a single one, that single hydrogen's peak will split into 'n+1' smaller peaks. For example, if a hydrogen atom is next to three other hydrogen atoms, its peak will split into four smaller peaks, which is called a quartet.
However, hydrogen atoms that are part of O-H or N-H bonds (like in water or some acids) usually don't split. Instead, they often appear as a wide, rounded bump on the diagram, called a "broad peak."
By carefully looking at all these details on the diagram, chemists can figure out exactly what compounds are in their sample. They can also check if the compound they made is pure and correct.
Images for kids
-
This is an NMR diagram for menthol. You can see how each hydrogen atom has a special shift, and how peaks split into smaller ones.
See also
 In Spanish: EspectroscopÍa de resonancia magnética nuclear para niños
In Spanish: EspectroscopÍa de resonancia magnética nuclear para niños
 | William Lucy |
 | Charles Hayes |
 | Cleveland Robinson |