Nitro compound facts for kids
A nitro compound is a special type of molecule that has a group of atoms called a nitro group. Think of a molecule as a tiny building block, and the nitro group is like a specific part attached to it.
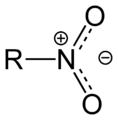
The nitro group is made of one nitrogen atom connected to two oxygen atoms. It looks like -NO2. This group is then attached to the rest of the molecule.
What are Nitro Compounds?
Nitro compounds are molecules that contain a special group of atoms called a nitro group. This group is made up of one nitrogen atom and two oxygen atoms. It's often written as -NO2. The nitrogen atom in this group is connected to the main part of the molecule.
Normally, a nitrogen atom likes to form three bonds. But in a nitro group, it forms four bonds, which gives it a positive charge. One of the oxygen atoms then has a negative charge, and the other has a double bond. Scientists often describe the nitro group as having "delocalized electrons." This means the electrons are shared across all three atoms (one nitrogen and two oxygens) in a special way.
Why are Nitro Compounds Important?
Many nitro compounds are known for being very explosive. This is because they can break apart very quickly when heated. When they break down, they release a lot of nitrogen gas. This reaction also gives off a huge amount of energy and heat.
A great example of an explosive nitro compound is trinitrotoluene, which is better known as TNT. The "nitro" part in its name tells you it contains nitro groups. Because of their explosive nature, nitro compounds are used in many different ways, from mining to making fireworks.
Images for kids
See also
In Spanish: Nitroderivado para niños
 | James Van Der Zee |
 | Alma Thomas |
 | Ellis Wilson |
 | Margaret Taylor-Burroughs |